Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Histomorphological and Neurobehavioural Effects of Aspartame on Weaned Wistar Rats
*Corresponding author: Oluwagbenga Oluwasemilore Joseph, CAC Ogbomoso DCC House, Ogbomoso, Oyo State, Nigeria.
Received: August 06, 2024; Published: August 16, 2024
DOI: 10.34297/AJBSR.2024.23.003122
Abstract
Aspartame is a white, odourless, powdered methyl ester comprised of aspartic acid and phenylalanine. It has been used for over 30 years as a food additive and it is also used in the pharmaceutical company in the production of anti-ulcer drug. This study is aimed to investigate the histomorphological and neurobehavioural effect of aspartame on the cerebellum of weaned Wistar rats.
Eighteen weaned Wistar rats weighing between 25g-50g were randomly divided into three groups (n=6). Group 1 (control) received 10 mg/kg bw of distilled water; Group 2 and 3 were administered 40 mg/kg bw and 80 mg/kg bw of aspartame respectively via oral route of administration. Neurobehavioural studies were carried out using standard behavioural tests after the first and last doses of aspartame. Brains were excised and fixed in 10% neutral buffered formalin to avoid autolysis. However, Sections of the cerebellar cortex were processed for routine paraffin embedding, cut at 5 µm and stained using hematoxylin and eosin for general histological study. Nonetheless, All the data were analyzed by analysis of variance (ANOVA) and post-hoc tests (Tukey HSD) used to determine source of a significant effect. Results were expressed as Mean ± S.E.M., p<0.05 is taken as accepted level of significant difference from vehicle or standard.
The results revealed a significant (p<0.05) reduction in the body weight of the rats, this may be due to oxidative damage. Also, from the novelty induced behaviour test carried out, it was observed that there was a significant (p<0.05) decrease in locomotor activities and significant (p<0.05) increase in rearing and grooming activities. There is also an increased in the number of open arms visited by the treated groups (group 2 and 3) when compared with the control group (group 1), this shows an increased level of anxiety in the treated groups (group 2 and 3) most specifically the group three with higher dosage (80 mg/kg bw) of aspartame. There’s also a greater neurodegeneration of the purkinje cells in group three with 80 mg/kg bw aspartame when compare with group two with 40 mg/kg bw.
The study concluded that administration of aspartame has a lot of neurotoxic effect on the neurobehavioral activities of the experimental animals and also neurodegeneration activities on the cells (purkinje cells) of the cerebellum at chronic dosage. Also, it causes increase in anxiety of the experimental animal. Further studies on the effect of aspartame on neurotransmitters of the brain and regulation of industrial usage of aspartame by the government are therefore recommended.
Keywords: Aspartame, Histomorphology, Neurobehaviour, Weaned, Necrosis
Introduction
Aspartame is a dipeptide of the amino acids aspartic acid and phenylalanine joined by a methyl ester [1]. It was discovered in 1965 by chemist James Schattler while using it in the development of an anti-ulcer drug and found it had a sweet flavour [2]. Hence, it has been widely used for over 30 years as a food additive due to its very strong, sweet taste. While having a similar caloric profile to sucrose, its intensity of sweetness renders calorie intake negligible [1]. It is slightly soluble in water [3]. However, the solubility increases with high and low pH and heating, but various types of degradation also occur- in particularly strong acidic or alkaline conditions, aspartame may be used to produce methanol, or free amino acids via hydrolysis [4].
Aspartame is a white, odourless, powdered methyl ester comprised of aspartic acid and phenylalanine; hence its IUPAC name N-(L-α-Aspartyl)-L-phenylalanine,1-methyl ester. It is ~200 times sweeter than sucrose and is found in a wide range of products, including diet sodas, confectionary foods, chewing gum, and medications [1]. It is composed of phenylalanine (50%) aspartic acid (40%) and methanol (10%) [5]. Phenylalanine plays an important role in neurotransmitter regulation, whereas aspartic acid is also thought to play a role as an excitatory neurotransmitter in the central nervous system [6]. Glutamate, asparagines and glutamine are formed from their precursor, aspartic acid [6]. Methanol is converted in the body to formate; which can either be excreted or can give rise to formaldehyde, diketopiperazine and other compound derivatives that are toxic [7].
It is mostly taken by people trying to lose weight or patients and children with diabetes. Although there is concern and research evidence suggesting possible adverse neurological and behavioural effects due to aspartame’s metabolic components (phenylalanine, aspartic acid, methanol and diketopiperazine) which are release during aspartame metabolizes [7]. However, Studies have shown that consumption of aspartame could cause neurological and behavioral disturbances in sensitive individuals [8]. Headache, insomnia and seizures are some of the disorders that have been encountered, and this may be accredited to changes in regional brain concentrations of the catecholamines released which are nor-epinephrine and epinephrine [9]. Therefore, the study aimed to investigate the histomorphological and neurobehavioural effect of aspartame in weaned rats.
Material and Method
Experimental animals
Eighteen (18) Weaned Wistar rats weighing between 25g-50g were used for the experiments. The rats were obtained from Laboratory Animal Department of Ladoke Akintola University of Technology. The experimental animals were acclimatized in the Anatomy Department, College of Health Sciences, Ladoke Akintola University of Technology, Ogbomoso for two weeks at room temperature with a 12hr light and dark cycle. They were fed with standard pellets gotten from the animal house and distilled water ad libitum. The rats received humane care according to the criteria outlined in the “Guidelines for the Use of Animals in Neuroscience and Behavioural Research” prepared by the Committee on Guidelines for the Use of Animals in Neuroscience and Behavioural Research; National Research council of the National Academies (2003).
Experimental Design
Eighteen (18) Weaned Wistar rats were randomly assigned into three (3) groups of six (6) rats each per group. Animals were administered vehicle, distilled water at 10 mg/kg, and aspartame at 40 and 80 mg/kg respectively for 21 days. Neurobehavioral studies were carried out using standard behavioural tests after the first and last doses of aspartame. The Open field was used to study novelty induced behaviours (locomotion, rearing and grooming) and elevated plus maze for anxiety. At the end of the experimental period, animals were euthanized by cervical dislocation. Brains were excised and fixed in 10% neutral buffered formalin. Sections of the cerebellar cortex were processed for routine paraffin embedding, cut at 5µm and stained using haematoxylin and eosin for general histological study.
Experimental Models
Novelty Induced Behaviour
The animals were exposed to the open field for about 10 min. During the period of exposure, rearing, grooming, and horizontal line crossing were recorded.
Learning Memory: Radial arm maze
The animals were exposed to the radial arm for about 5 min and the appropriate movement of the rats into the various arms were recorded.
Anxiety Model: Elevated PlusMaze
The animals were exposed to the elevated plus maze for about 5 min. The numbers of time the animal visited the open arm of the elevated plus maze were recorded.
Organ’s Preparations
The animals were sacrificed on the 21st day of administration using cervical dislocation method. The incisions were made on the head and the cerebellum specimen was harvested and weighed across all groups. The cerebellum specimen was fixed using Bouin’s fluid and subjected into hematoxylin and eosin (H & E).
Tissue Processing
The harvested cerebellum specimens were fixed using Bouin’s fluid to arrest autolysis. The tissues were removed and rinsed in tap water before being run through ascending grades of alcohol for dehydration. The tissues were taken through two (2) changes of xylene followed by tissue infiltration by a suitable histological wax. The tissues were transferred into two (2) changes of bath containing molten paraffin wax in a chamber of automated embedding machine for 1-2 hours each. The embedding was performed in special container called cassette which help to give shape to the wax containing the tissue when it is set. The tissue blocks were removed and air dried until the wax became solidified followed by sectioned with a microtome machine. The tissue samples were stained with hematoxylin and eosin (H & E) routine staining.
Photomicrography
Brain sections were examined under an Olympus trinocular microscope (XSZ-107 E, Japan) with a digital camera (Canon powershot 2500) attached.
Statistical Analysis
All the data were analyzed by analysis of variance (ANOVA) and post-hoc tests (Tukey HSD) used to determine source of a significant effect. Results are expressed as Mean ± S.E.M., p<0.05 is taken as accepted level of significant difference from vehicle or standard.
Results and Discussion
Results
Figure 1 shows the effect of aspartame on body weight measured as percentage weight change mean. Percentage weight change is defined as the difference between the final and initial body weights divided by initial weight multiplied by 100. There was a significant (F=30.14, p<0.05) decrease in body weight gain at 80 mg/kg of aspartame compared to vehicle.
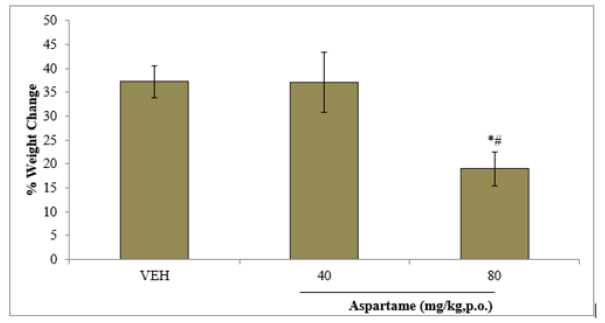
Figure 1: Effect of aspartame on % weight change. Each bar represents Mean ±S.E.M, n=6, *p<0.05 vs. VEH. VEH: vehicle.
A. EFFECT OF ASPARTAME ON LOCOMOTOR ACTIVITY
Following 10 minutes of exposure to the open field, there was a significant (F=11.53, p>0.05) decrease in locomotor activity at both doses of aspartame tested compared to vehicle. There was also a significant decrease in locomotor activity following sub chronic compared to acute aspartame administration at 40 mg/kg but not at 80 mg/kg as shown in Figure 2.
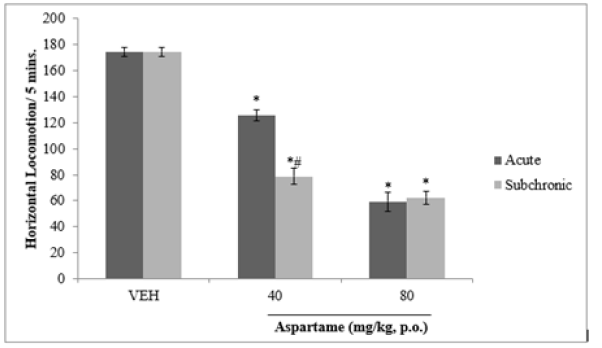
Figure 2: Effect of aspartame on locomotor activity. Each bar represents Mean ±S.E.M, *p < 0.05 vs. vehicle, #p< 0.05 acute vs. subchronic, n=6.VEH: vehicle.
B. EFFECTS OF ASPARTAME ON REARING ACTIVITY
Following 10 minutes of exposure to the open field, there was a significant (F=15.27, p<0.05 increase in rearing activity in at 40 and 80 mg/kg of aspartame compared to vehicle following acute administration and npo significant difference with sub chronic administration as shown in Figure 3.
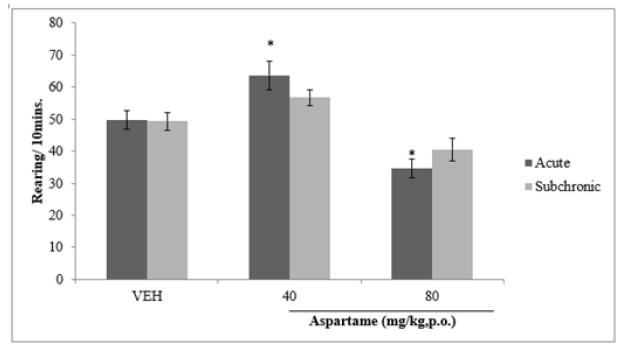
Figure 3: Effect of aspartame on rearing activity. Each bar represents Mean ±S.E.M, *p < 0.05 vs. VEH, n=6. VEH: Vehicle.
C. EFFECT OF ASPARTAME ON GROOMING BEHAVIOUR
Following 10 minutes of exposure to the open field there was a significant (F=25.54, p<0.05) increase in grooming behaviour at both doses of aspartame compared to vehicle following both acute and subchronic administration as shown in Figure 4.
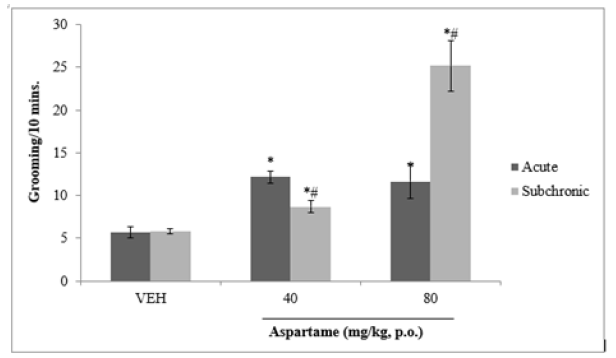
Figure 4: Effect of aspartame on grooming behaviour. Each bar represents Mean ±S.E.M, *p < 0.05 vs.VEH, #p < 0.05 acute vs. subchronic, n=6. VEH: Vehicle.
D. EFFECT OF ASPARTAME ON ANXIETY RELATED BEHAVIOUR
Figure 5 shows effect of aspartame on anxiety related behaviours measured by percentage number of open arm visits. There was a significant increase in number of open arm visits at 40 mg/kg of aspartame compared to vehicle following acute administration. At 40 (acute) and 80 mg/kg (acute and sub chronic) no significant difference was seen.
E. EFFECT OF ASPARTAME ON MEAN RELATIVE BRAIN WEIGHT
Figure 6 shows the effect of aspartame on the mean relative brain weight. There was no significant difference in brain weight at any dose of aspartame compared to vehicle.
F. THE EFFECT OF ASPARTAME ON THE HISTOLOGY OF THE CEREBELLAR CORTEX
(Plates 1-3).
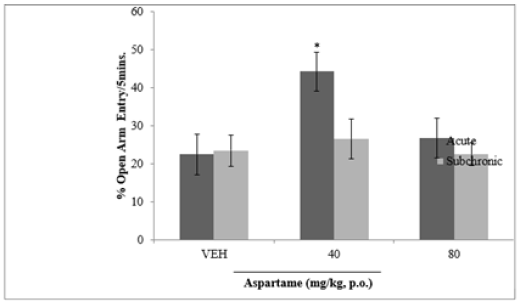
Figure 5: Effect of aspartame on anxiety related behaviours. Each bar represents Mean ±S.E.M, n=6; VEH: vehicle.
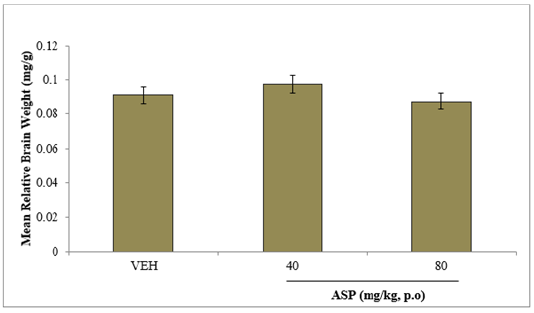
Figure 6: Effect of aspartame on relative brain weight. Each bar represents Mean ±S.E.M, n=6; VEH: vehicle.
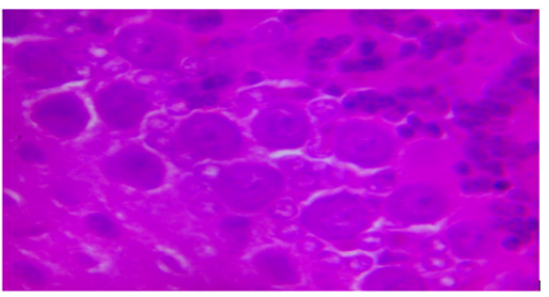
Plate 1: Photomicrograph of cerebellar cortex of rats administered aspartame at 40mg/kg showing purkinje cells and granule cells. H&E X400.
Discussion
Effect of Aspartame on Body Weight
The significant reduction in the body weight of the animal maybe due to oxidative damage. This is in agreement with Shaher, et al. [4] who reported that oxidative damage caused marked body weight loss in rats upon methanol intoxification. Also, formaldehyde which is a first metabolite of methanol, increase the population of shrunken cells, dead cells, hydolipid cells [10].
Effect of Aspartame on Novelty Induced Behaviours (locomotion, rearing and grooming)
From the novelty induced behavior test carried out, it was observed that there was a significant decrease in locomotor activities and significant increase in rearing and grooming activities. The significant decrease observed in the locomotor activities may be due to loss of dopamine from the brain system as seen in Parkinson disease [9,11]. From the metabolism process of aspartame, phenylalanine one of the main components of aspartame, competes with tyrosine for neutral amino acid transporter (NAAT), this result to a compromised dopamine production because phenylalanine will bind with NAAT more frequently than tyrosine resulting to high concentration of phenylalanine and lower concentration of dopamine resulting to impaired motor function of the brain. This result also correlates with the findings of Rachna, et al. [12].
The increase in rearing and grooming in the experimental animal compared to the control animal may be as a result of memory loss by the animals. Memory loss in aspartame induced rats is thought to be due to aspartic acid and phenylalanine without the other amino acid found in protein. These neurotoxic agents might cross the blood brain barrier and deteriorate the neurons of the brain. This report supports previous studies that have shown that chronic consumption of aspartame produces impairment in learning and memory processes [13].
Effect of Aspartame on Anxiety Related Behaviours
Increase in the number of open arm visited by the rats shows an increase level of anxiety. 50% phenyalanine in aspartame, which is neurotoxic goes directly into the brain and lowers the seizure threshold leading to depletion of serotonin. Once the level of serotonin is depleted, it triggers anxiety, manic depression or bipolar and mood swings. This correlates with the discussion of Marte and Aurora [6] and Rachna, et al. [12].
Effect of Aspartame on Cerebellar Morphology (causes purkinje cell death)
Oral intake of Aspartame in mice has been reported to be the cause of neuronal necrosis in several regions of the brain including the hypothalamus [7,14]. Histological observation of the cerebella cortex shows neurodegeneration of the purkinje cells at aspartame dosage of about 80kg/bw. The neurodegeneration of the purkinje cells might be as a result of activities of aspartame on N-Methyl-D-Aspartate (NMDA) receptors. Aspartame may act on NMDA receptors, leading to continuous activation of these receptor sites resulting in no binding space for glutamate, in which continuous activation of these sites might cause damage to the brain neurons as described by Sara, et al. [8] and Choi and Rothman [15]. This also supports the works of David, et al. [16] that aspartame acts as an agonist of glutamate on the NMDA receptor.
Conclusion
From the result of the study carried out, it can be deduced that administration of aspartame has a lot of neurotoxic effect on the neurobehavioral activities of the experimental animals and neurodegeneration activities on the cells (purkinje cells) of the cerebellum at chronic dosage. Also, it causes an increase in anxiety of the experimental animal. Further studies on the effect of aspartame on neurotransmitters of the brain and regulation of industrial usage of aspartame by the government are therefore recommended.
Declarations
Ethics approval
The animals used were in accordance with the rules and guidance of Ladoke Akintola University of Technology, Faculty of Basic Medical Sciences, Ogbomosho, Oyo State, Nigeria.
Data Availability Request
The data generated during the study will be provided on a reasonable request from corresponding author.
Declaration of interests Statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Funding Statement
There was no particular grant for this research from any funding body in the public, private, or any otherwise domains.
References
- More TA, Sheikh Z, Ali A (2021) Artificial Sweeteners and their Health Implications: A Review. Biosci Biotech Res Asia 18(2).
- Syed S AI, Haleema N, Namra N (2023) Historical and current perspectives on the human consumption of non-nutritive sweeteners (NNS). JAMDC 5(4): 243-249.
- Jiasi Yu, Wei Hu, Dan Wu, Yun Ding, Xin Wang, et al. (2022) Determination of aspartame and alitame in liquid dairy products and milk-containing beverages in the Chinese market. Italian Journal of Food Science 34 (3): 91-98.
- Shaher SAA, Mihailescu DF, Amuzescu B (2023) Aspartame Safety as a Food Sweetener and Related Health Hazards. Nutrients 15(16): 3627.
- Choudhary AK, Lee YY (2018) Neurophysiological symptoms and aspartame: What is the connection? Nutr Neurosci 21(5): 306-316.
- Marte I Flydal, Aurora Martinez (2013) Phenylalanine hydroxylase: function, structure, and regulation. IUBMB life 65 (4): 341-349.
- Marinovich, More TA, Sheikh Z, Ali A l (2013) Aspartame, low-calorie sweeteners and disease: regulatory safety and epidemiological issues. Food and Chemical Toxicology 60: 109-115.
- Sara K Jones, Deirdre M McCarthy, Cynthia Vied, Grgg D Stanwooda, Chris Schatschneiderc, et al. (2022) Transgenerational transmission of aspartame-induced anxiety and changes in glutamate-GABA signaling and gene expression in the amygdala. Proceedings of the National Academy of Science 119(49): 2213120119.
- Choudhary AK, Lee YY (2018) The debate over neurotransmitter interaction in aspartame usage. Journal of clinical neuroscience 56: 7-15.
- Nakao H, Umebayashi C, Nakata M, N ishizaki Y, Noda K, Okanao Y, at el. (2003) Formaldehyde induced shrinkage of rat thymocyte. J. Phamacol. Sci. 91(1): 83-86.
- Kolb B, Whishaw IQ (2003) Fundamentals of human neuropsychology (5th ed.). Worth Publishers.
- Rachna Manek, Yao V Zhang, Patricia Berthelette, Mahmud Hossain, Cathleen S Cornell, et al. (2021) Blood phenylalanine reduction reverses gene expression changes observed in a mouse model of phenylketonuria. Scientific Reports 11(1): 22886.
- Collison K S, N J Makhoul, M Z Zaidi, Soad M Saleh, Bernard Andres, et al. ( 2012) Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS One 7(4): 1-13.
- Olney JW, J Labruyere, T de Gubareff (1980) Brain damage in mice from voluntary ingestion of glutamate and aspartate. Neurobehav Toxicol 2: 125-129.
- Choi DW, Rothman S M (1990) The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu. Rev. Neurosci. 13: 171-182.
- David M Wilson, Mark R Cookson, Ludo Van Den Bosch, Henrik Zetterberg, David M Holtzman, et al. (2023) Hallmarks of neurodegenerative diseases. Cell, 186 (4): 693-714.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.