Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Assessing the Prevalence and Antibiotic Resistance of Staphylococcus aureus in Powdered Milk Sold in Owerri Municipal and Surrounding Areas: Implications for Food Safety and Public Health
*Corresponding author: Charity Ndidi Obum-Nnadi, Department of Medical Laboratory Science, Faculty of Nursing and Allied Health Sciences, University of Abuja, Nigeria.
Received: August 26, 2024; Published: September 20, 2024
DOI: 10.34297/AJBSR.2024.24.003167
Abstract
Milk, a nutritious food source, can support the growth of various microbes, including Staphylococcus aureus. This study investigates the prevalence and antibiotic resistance patterns of Staphylococcus aureus in powdered milk sold in Owerri Municipal and surrounding areas. A total of 50 exposed powdered milk samples were collected from different vendors at retail points in Relief Market. The samples were analyzed for Staphylococcus aureus using cultural methods, with identification based on colonial morphology, microscopy, and biochemical tests. The antibiotic susceptibility of the isolates was assessed using the Kirby-Bauer disc diffusion method. Results indicated that the highest mean Staphylococcus aureus count was 1.44 x 10^4 CFU/g in samples exposed outside, while the lowest was 1.19 x 10^4 CFU/g in samples stored inside. Out of the 50 samples, 15 tested positive for Staphylococcus aureus, representing a 30% prevalence rate. Samples exposed outside had a higher occurrence (40%) compared to those stored inside (20%). The antibiogram revealed that the isolates were highly susceptible to Ciprofloxacin (100%), Gentamicin (93.33%), Erythromycin, and Chloramphenicol (73.33%). However, resistance was observed against Norfloxacin (66.67%) and Ampicillin-cloxacillin (26.67%). Notably, five isolates exhibited multi-drug resistance, with a Multiple Antibiotic Resistance Index (MARI) ranging from 0.3 to 0.4. The study highlights a significant prevalence of Staphylococcus aureus in powdered milk and its resistance to commonly used antibiotics, posing a potential public health risk to consumers.
Keywords: Staphylococcus aureus, Antibiotic resistance, Enterotoxins, Pathogens, Milk
Introduction
Milk is a vital nutrient-rich food essential for human growth and maintenance [1]. However, it also serves as an ideal medium for microbial growth. The microbiological quality of milk can be influenced by several factors, including the composition of the initial raw milk flora, processing conditions, and potential contamination during packaging or handling after heat treatment [2]. A wide range of microorganisms can cause spoilage in milk and dairy products, including lactic acid bacteria, coliforms, Gram-negative psychrotrophs, molds, and yeasts. Additionally, public health concerns include pathogenic bacteria such as Campylobacter jejuni, Listeria monocytogenes, Salmonella spp., pathogenic strains of E. coli, Yersinia enterocolitica, and enterotoxigenic Staphylococcus aureus [3,4]. Since the early days of the dairy industry, pathogenic bacteria in milk and dairy products have been a significant public health issue, as these pathogens can lead to various diseases when these products are consumed (Van Kessel, et al., 2014).
The potential of bacteria to cause foodborne illnesses depends on their ability to produce toxins after ingestion or on the presence of preformed toxins in food. Staphylococcus aureus is a notable pathogen in this context, leading to gastroenteritis from consuming contaminated food. Food poisoning caused by Staphylococcus aureus is attributed to the ingestion of enterotoxins that are performed in the food (Loir et al., 2016). It is recognized as the third leading cause of foodborne illnesses globally, with milk, dairy products, and meats-especially those that are improperly handled-being major sources [8]. Consumption of unpackaged powdered milk poses a health risk to consumers as the milk stands a high risk of contamination through nasal passages, frequent contact with hands via which S. aureus can be introduced by being a normal flora of the body [5].
Powdered milk can occasionally be contaminated with pathogenic microbes, but due to its low moisture content, these microbes typically do not grow. However, they can remain viable for extended periods. While these microbes may not directly cause spoilage, if the powdered milk is improperly rehydrated and exposed to time-temperature abuse, the microorganisms can multiply and potentially cause illness. Therefore, monitoring the presence of these pathogens is crucial, as it indicates the hygienic standards maintained throughout production, processing, packaging, handling, and retailing (Wouters, et al. 2015).
Staphylococcus aureus is associated with numerous diseases in both animals and humans, with its pathogenicity stemming from a combination of genetic factors that enhance its virulence, invasiveness, immune evasion, and antibiotic resistance [6]. Milk and dairy products, particularly those produced from raw milk under poor hygienic conditions, can be significant vehicles for the spread of foodborne pathogens, including antibiotic-resistant strains of S. aureus (Kadariya, et al., 2014). In the United States, S. aureus is responsible for approximately 241,000 cases of food poisoning annually [6]. This pathogen is among the leading causes of foodborne disease outbreaks worldwide, contributing to a range of illnesses and conditions (Jamali, et al., 2015).
Milk is a significant source of staphylococcal food poisoning, with numerous documented foodborne outbreaks linked to the consumption of contaminated milk (Fetsch, et al., 2014). Additionally, raw milk and raw milk products often harbor various strains of S. aureus globally (Jamali, et al., 2015; Riva, et al., 2015). Milk provides an ideal environment for S. aureus to grow and produce enterotoxins, which can remain biologically active even after pasteurization [7] (Rall, et al., 2018).
Materials and Methods
Study Area
This study was conducted in Imo State, which is located in the southeastern region of Nigeria with a population size of 4.8 million people and a population density of between 230-1400 people per square kilometer. The state is situated in the south-eastern vegetation belt of the country and lies between latitudes 5° 4 and 6° 3 N and longitudes 6° 15 and 7° 34 E. Owerri is a big city located in Imo State, Nigeria and is the capital of Imo State.
Materials
The materials used were powdered milk, Nutrient Agar, Mannitol salt agar, Mueller-Hilton agar, distilled water, oxidase reagent, Gram stain kit, mannitol, glucose, lactose and sucrose, incubators, pipette, petri dish, refrigerator, autoclave, colony counter, light microscope, balance (weights; 2014 g capacity), petri dishes (90x15), sterile cups (100 ml), syringes (1,5 and 10 ml), needles, forks, spatulas, sterile forceps, sterile 1-shaped glass rods, sterile flasks, inoculating loops and ice box.
Collection of Samples
A total of 50 exposed powdered milk samples were collected from different vendors at retail point in Relief market. The samples were packaged in a clean polythene bag, labelled appropriately and transported to the Microbiology Laboratory, Imo State University for further processing and microbiological analysis
Microbiological Analysis
Sterilization Technique: The materials used for this study were sterilized using standard techniques. Glass wares were sterilized in the hot air oven at 160C for 1 hour. Culture media were sterilized by autoclaving at 121 15psi pressure unit for 15 minutes. Inoculation wire loop was sterilized by flaming intermittently to red hot over a Bunsen flame. Glass rod spread (hockey stick) was sterilized intermittently by dipping in absolute alcohol and bringing it over a burning flame to burn off. Bench top, inoculation hood and working area were sterilized by disinfecting with purit antiseptic and covering with 75%ethanol. Sterile disposable hand gloves and face mask were worn and changed after each procedure to ensure aseptic conditions.
Preparation of Media
The media were of analytical grade (OxoidTM, UK) and they included Mannitol Salt Agar (MSA), nutrient agar (NA), Mueller-Hinton agar and buffered peptone water. All the media were prepared according to the manufacturers' instructions.
Enumeration and Isolation of Staphylococcus aureus: This was carried out according to the procedure decribed by [8]. Twenty-five (25g) of the powdered milk was added to 225mL of sterile normal saline and mixed thoroughly. A tenfold serial dilution to 10-5 was carried out, and 0.1mL of the 10-2 dilution was spread-inoculated onto mannitol salt agar. The plates were incubated aerobically at 37 for 24hours. Appearances of golden yellow colonies were presumptively considered to be Staphylococcus aureus. The observed colonies characteristics of S. aureus were counted. All counts were expressed as Colony Forming Units Per gram (CFU/g), Colonies were picked and stored on nutrient agar slants for further confirmation tests.
Characterization of Isolates
Presumptive Staphylococcus species were Gram-stained and the Gram-positive cocci in clusters were subjected to biochemical characterization using catalase and coagulase tests as described by [9].
Gram Staining
The pure isolates were stained according to Gram's techniques as described by Baker. This consists of the following steps:
A thin smear was prepared on clean glass slide, air-dried, and heat-fixed by placing the slide gently over the flame of the spirit lamp. The smear was stained with crystal violet for 1 minute, and then rinsed with tap water. The smear was covered with Lugol's iodine for 60 seconds and washed off under gentle running tap water. The slide was decolorized using 70% ethanol after which was then washed under tap water and then counterstained with safranin for 30 seconds. It was rinsed again with tap water and the slide blotted dry with a piece of filter paper. The stained cells were examined with the oil immersion objective lens of the light microscope. The gram-positive organisms were characterized by a purple colour while a gram-negative organism took on a pink colour. Biochemical Tests
Catalase Test
Catalase test was performed to distinguish catalase-negative Streptococcus spp from catalase-positive Staphylococcus spp using a microscopic slide. 5% of hydrogen peroxide was added to a small sample of the isolate on the slide. Formation of bubbles on the slide confirmed the isolate to be Staphylococcus spp while absence of bubbles confirmed that the isolates were not Staphylococcus spp. Staphylococcus spp are catalase positive.
Coagulase Test
Isolates were inoculated into test tubes containing 0.5 ml of rabbit plasma or human plasma. After mixing by gentle rotation, the tubes were inoculated at 37 along with a negative control tube containing a mixture of 0.5ml of sterile Tryptone Soya Broth (TSB) and 0.5ml of rabbit plasma or human plasma. Clumping was evaluated at 30 minutes intervals for the first 4hrs of the test and then after 24hrs of incubation. They were allowed to stand undisturbed (Talaro, et al., 2005). Coagulation was verified after incubation.
The isolates were considered coagulase positive if they exhibit some levels of coagulation. This test was used to confirm that the Staphylococcus spp confirmed from the catalase test is Staphylococcus aureus.
Antimicrobial Susceptibility Testing
The antibiotic susceptibility tests for the isolates were performed by Kirby-Bauer (disc diffusion) method. Isolates were grown on nutrient agar for 24 hours and colonies suspended in 2 mL sterile normal saline until a turbidity equivalent to 0.5 MacFarland standards was obtained. A 0.1 mL aliquot of each bacterial suspension was separately inoculated on freshly prepared Mueller-Hinton agar with the aid of sterile swabs to form a bacterial lawn (Okpo, et al., 2016). Standard antibiotic discs were placed on the inoculated plates equidistant from each other and pressed gently using sterile forceps to ensure contact with the agar surface. The plates were allowed to stand for 15 minutes pre-diffusion time and then incubated aerobically at 37° C for 24 hours. The diameter of zones of inhibition were measured to the nearest millimetre and the isolates were characterized as susceptible, intermediate or resistant to the antibiotic used in reference to the interpretation standards provided by [10]. The antibiotics used were Norfloxacin (30ug); Chloramphenicol (30μg); Erythromycin (10μg); Ciprofloxacin (10μg); Gentamicin (10μg); Streptomycin (30μg); and Ampicillin-cloxacillin (20μg).
Results
The exposed powdered milk samples obtained were analysed for their microbial and Staphylococcal count profile. The mean Total Viable Count (TVC) ranged from 1.32×104 − 2.10×CFU / gCFU / g CFU/gCFU/g. Staphylococcal count of the powdered milk samples was determined, with samples displayed outside the shop in sacks having the highest mean count (1.44×104CFU / g). Whereas the least mean count (1.44×104CFU / g) was observed in samples displayed inside the shop in sack (Table 1).
Out of the total of 50 exposed powdered milk samples examined, fifteen isolates were confirmed to be Staphylococcus aureus based on colonial, microscopic and biochemical characteristics (Table 2).
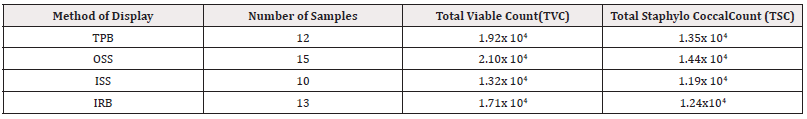
Table 1: Mean Total Aerobic Microbial Load of Exposed Powdered Milk Samples in Relation to Methods of Display (CFU/g).
*Key Points: TPB: Tied in a polythene bag; OSS: Outside the shop in a sack; ISS: Inside the shop in a sack; IRB: Inside a rubber bucke; CFU/g: Colony forming unit per gram.
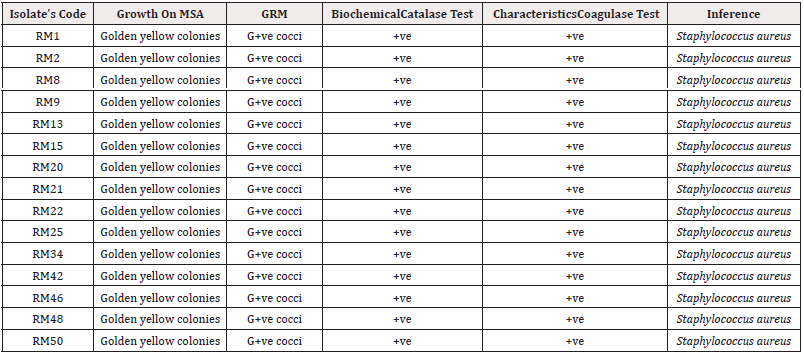
Table 2: Cultural, Microscopic and Biochemical Characterics of Staphylococcus Isolate.
*Key Points: RM: Relief Market; MSA: Mannitol Salt Agar; GRM: Gram Reaction and Morphology; G+ve: Gram-positive; +ve: positive.
An overal1 percentage occurrence of 30% of Staphylococcus aureus was observed in this study, with samples displayed outside the shop in sacks having the highest occurrence of 40% while the least (20%) observed in those displayed inside the shop in sacks (Tables 3,4).
Discussion
Over the years, milk and milk products have been known as vehicles for the transmission of bacterial pathogens to man (Revathi, et al., 2014). The contamination of food with antibiotic-resistant pathogens poses a significant global public health threat. The determinants of antibiotic resistance in these pathogens can be transferred to other bacteria of clinical significance.
The Staphylococcal counts of the powdered milk samples examined in this study with respect to their methods of display at retail point revealed high counts. These high counts observed for all the samples irrespective of their methods of display might be attributed to post-processing contamination due to low hygienic practices of the retailers. This might have led to the introduction of staphylococci into the products via direct contact by hands or respiratory secretions as the organism is a normal flora of the skin, throat, and nasal passages. The staphylococcal count of the powdered milk samples exceeded the satisfactory limit of < 102 CFU/g by the Food Standards of < 102 Australia New Zealand (2018). Afroz, et al. (2015) also reported staphylococcal range counts of 1.5×102 − 3.6×103 CFU / g in full cream powdered milk sold under markets of 1.5×102 − 3.6×103CFU / g conditions in Dhaka, Bangladesh. A high percentage occurrence of 30% of Staphylococcus aureus was observed in this study. This indicates that consumption of this contaminated powdered milk has serious health implications [11-19].
Recommendation
There is need for effective control measures to safeguard public health from this food borne pathogens. This can be achieved through creating awareness to food vendors to adhere strictly to personal hygiene and avoid direct contact with the product especially when measuring. Potential health risks to the consumers and may be a possible source of food borne illnesses for the consumers of these products in and around the study location.
Resistance of pathogenic microbes against antibiotics is a major concern globally, as it leads to failures in treatment of human and animal diseases. The Staphylococcus aureus isolates showed high susceptibilities to ciprofloxacin, gentamicin, erythromycin, and chloramphenicol. These observed susceptibilities might be due to their molecular sizes, a factor which increases their solubility in diluents and consequently further their penetration ability through cell wall into the cytoplasm of the target organism (Mailard, 2014; Poole, 2014). However, the isolates were observed to be highly resistant to norfloxacin, and moderately resistant to Ampicillin-cloxacillin. The resistance observed could be attributed to antibiotic abuse inform of overuse and misuse of antibiotic. The antibiotic susceptibility patterns observed in this study is similar to the observations reported by Okpo, et al. (2016).
MAR index observed in this study ranged between 0.3-0.4. A MAR index greater than 0.2 shows a high-risk source of contamination where antibiotics are frequently used (Furtula, et al., 2015). The MAR index observed among the Staphylococcus aureus isolates might be due to over-the-counter usage of antibiotics, and self-medication.
Conclusion
An overall occurrence of 30% of Staphylococcus aureus from powdered milk was observed in this study, and this poses a health threat for the consumers of this product. Antibiogram of the Staphylococcus aureus isolates revealed high susceptibilities to ciprofloxacin, gentamicin, erythromycin, and chloramphenicol for effective treatment where necessary.
Conflict of Interest Statement
None.
Acknowledgement
None.
References
- Bashar T, Malek MA (2016) Prevalence of microbial contaminants of some milk and milk products available in different markets of Dhaka. Bangladesh Journal of Microbiology 23(1): 75-77.
- Ahmed S, Anwar MN (2016) Microbial Contaminants of Dried Powder Milk Available in Local Markets of Bangladesh. Bangladesh Journal of Microbiology 23(2): 162-164.
- Agboke AA, Osonwa UE, Opurum CC and Ibezim EC (2015) Evaluation of microbiology quality of some soybean milk products consumed in Nigeria. Prime Research on Medicine 1(2): 25-30.
- Ateba CN, Mbewe M, Moneoang MS, Bezuidenhout CC (2014) Antibiotic-resistant Staphylococcus aureus isolated from milk in the Mafikeng Area, Northwest province, South Africa. South African Journal of Science. 106(11/12): 1-6.
- Argudin MA, Mendoza MC, Rodicio MR (2010) Food poisoning and Staphylococcus aureus enterotoxins. Toxins 7(2): 1751-1773.
- Scallan KY, Howden BP, Jiang JH, Stinear T and Peleg AY (2014) Population genetics and the evolution of virulence in Staphylococcus aureus. Infect Genet Evol 21: 554-562.
- Asao T, Kumeda Y, Kawai, T Shibata T, Oda H, et al. (2003) An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol Infect 130(1): 33-40.
- Aycicek H, Cakiroglu S, Stevenson TH (2005) Incidence of Staphylococcus aureus in ready-to-eat meals from military cafeterias in Ankara, Turkey. Food Control 16(6): 531-534.
- Afroz H, Sultana F, Fakruddin M, Kamrunnahar, Khan ZUM, et al. (2013) Isolation of Escherichia coli and Staphylococcus aureus from full cream powder milk sold under market conditions at Dhaka, Bangladesh and their antibiotic susceptibility. Journal of Advanced Scientific Research 4(3): 27-31.
- Arcuri EF, Angelo FF, Martins Guimaraes MF, Régine Talon, Maria de Fatima Borges, et al. (2014) Toxigenic status of Staphylococcus aureus isolated from bovine raw milk and Minas frescal cheesesin Brazil. Journal of Food Protection 73(12): 2225-2231.
- Ayele Y, Gutema FD, Edao BM, Girma R, Tufa TB, et al.(2017) Assessment of Staphylococcus aureus along milk value chain and its public health imortance in Sebeta, central Oromia, Ethiopia. BMC Microbiology 17(1): 141.
- Balaban N, Rasooly A (2000) Staphylococcal enterotoxins. International Journal of Food Microbiology 61(1): 1-10.
- Bennett SD, Walsh KA, Gould LH (2016) Foodborne disease outbreaks caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus Aureus-United States, 2018-2018. Clinical Infectious Diseases 57: 425-433.
- Bunning VK, Lindsay JA, Archer DL (1997) Chronic health effects of microbial foodborne disease. World Health Statistics Quarterly 50(1-2): 51-56.
- Byrd Bredbenner C, Berning J, Martin Biggers J, Quick V (2013) Food safety in home kitchens: a synthesis of the literature. International Journal of Environmental Research and Public Health 10(9): 4060-4085.
- Chaibenjawong P, Foster SJ (2011) Desiccation tolerance in Staphylococcus aureus. Archives of Microbiology 193(2): 125-135.
- Clinical and Laboratory Standards Institute (CLSI) (2015 Performance standards Foranti microbial susceptibility testing; twenty-fifth informational supplement M100-S25 35(3): 64-71.
- Cohen ML (2000) Changing patterns of infectious disease. Nature 406(6797): 762-767.
- De Buyser ML, Dilasser F, Hummel R and Bergdoll MS (2017) Enterotoxin and toxic shock syndrome toxin-1 production by staphylococci isolated fromgoat's milk. International Journal of Food Microbiology 5(4): 301-309.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.