Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Novel, Artificial Intelligence-based Approaches to the Mitigation and Treatment of Post-Traumatic Stress Disorder (PTSD) in Affected Populations
*Corresponding author: Jonathan RT Lakey, GATC Health Inc., 2030 Main Street, Suite 660, Irvine, CA, Departments of Surgery and Biomedical Engineering, University of California Irvine, Irvine, CA.
Received: December 15, 2024; Published: December 18, 2024
DOI: 10.34297/AJBSR.2024.25.003303
Keywords: Artificial Intelligence, Post traumatic stress disorder, PTSD, AI
Introduction
Post-Traumatic Stress Disorder (PTSD) is a prevalent chronic heterogenous condition with over half of a million symptom combinations meeting diagnostic criteria [1]. Individuals may have intense, disturbing thoughts and feelings related to their experience that persist long after such events lead to physiological dysregulation including alterations in neural circuitry, molecular biology, endocrinology, and immune response [2]. Furthermore, while PTSD commonly follows exposure to real or threatened traumatic events coupled with physical or emotional harm, high rates of co-morbidities such as substance use disorders complicates diagnosis and treatment [3]. Physiological dysregulation profoundly impacts individual and family functioning, and has been linked to significant medical, financial, and social problems. Consequently, novel interventions, including innovative therapeutic medications to treat and mitigate PTSD are highly warranted. Further, combining effective pharmacotherapy with psychotherapy modalities (e.g., Cognitive Behavioral Therapy (CBT) and Immersion Therapy (IT)) to elicit” systematic desensitization” represents a pressing, unmet need [4].
Epidemiological Context
Exposure to traumatic events and consequent PTSD is a pervasive human experience with a lifetime prevalence of approximately 6% in trauma-exposed individuals and about 5% of Americans have PTSD in any given year. However, military personnel, especially veterans, have an approximate 3-fold greater prevalence, reaching a prevalence of nearly 20% [5]. Moreover, veterans who deployed to a war zone are also more likely to have PTSD than those who did not deploy, females are more affected than males and younger veterans (6). The highest average prevalence among veterans was in 2021, with an overall prevalence of 24% among male veterans and 25% among female veterans, respectively, who had served in Afghanistan and/or Iraq [7]. There is also a correlation between Traumatic Brain Injury (TBI) and PTSD. Multiple studies have shown that injured combat personnel demonstrated a PTSD prevalence as high as 32%, when compared to 14% among uninjured personnel, even after correcting for pre-existing PTSD [8]. Moreover, the highest prevalence of PTSD was observed among injured soldiers who had lost consciousness. Similar studies in the U.K. among civilians also showed an increased prevalence of PTSD in patients with TBI [9]. Thus, a focus on military contexts, where PTSD rates range from 10- 20% depending on combat exposure, a comprehensive understanding of neuroendocrine circuitry underlying hyperarousal, intrusive memories, and emotional dysregulation significantly impacting quality of life and operational readiness are highly warranted.
Physiological Context
Given the integral stress-response mechanistically underpinning PTSD, fear neurocircuitry and alterations in neuroendocrine systems such as the hypothalamic-pituitary-adrenal (HPA) axis represent a core construct. The HPA axis regulates the hormonal stress response by releasing cortisol, a glucocorticoid hormone, following activation by corticotropin-releasing hormone (CRH) and adrenocorticotropin (ACTH) [10]. Cortisol exerts effects on the brain, including areas like the amygdala, hippocampus, and prefrontal cortex, to influence stress-responsive behaviors and physiological processes such as immune function and glucose regulation. In healthy individuals, cortisol’s negative feedback on the hypothalamus and pituitary gland ensures that the HPA axis returns to a resting state after acute activation, maintaining homeostasis. Emerging research and advancements in molecular technologies have allowed a more comprehensive understanding that psychological outcomes, neuroendocrine, and immune alterations extend beyond the HPA. The HPA axis is regulated by various neurotransmitters and neuropeptides, including catecholamines such as epinephrine and norepinephrine, which are released during stress to activate physiological responses such as increased heart rate and respiration [11]. Glutamate and GABA also play key roles, with GABA exerting inhibitory control to maintain homeostasis, while stress-related increases in glutamate can overcome this inhibition, affecting brain regions like the prefrontal cortex and hippocampus. Serotonin (5- HT) regulates functions such as mood, sleep, and appetite and is implicated in PTSD, with stress elevating serotonergic activity and influencing cortisol and ACTH levels. While monoamines and catecholamines may serve as biomarkers for PTSD symptoms, their utility in therapeutic interventions is limited, as first-line SSRIs provide only moderate relief and fail to address the full spectrum of symptoms.
Genetic Context
The genetics of PTSD involve complex interactions between multiple genes and environmental factors. Genes related to the HPA axis, serotonin, dopamine, and neuroplasticity, (e.g., FKBP5, SLC6A4, and BDNF), have been implicated in increasing susceptibility to PTSD, often influenced by trauma exposure and epigenetic modifications. Further, heritability of PTSD susceptibility can now be detected by sex-dependent SNPs (single nucleotide polymorphisms) and is estimated to range between 5–20%. Genome-wide loci have been identified in the Veterans Administration Million Veteran Program (VA-MVP), using >200,000 subjects in a genome-wide association study (GWAS). The study compared quantitative trait loci (QTL) differences between European and African ancestry [12]. Findings also demonstrate that there are more discernable biomarker loci in men than women [13]. Statistically, major depressive disorder (MDD), diabetes, and cardiovascular diseases are among the major clinical comorbidities associated with PTSD patients as are other psychiatric disorders such as schizophrenia [14]. The VAMVP also demonstrated that polygenic risk for PTSD is significantly predictive of re-experiencing symptoms, clinically known by the moniker “REX.” It has also been reported that PARK2, a dopamine (DA) regulatory gene, is associated with PTSD [15]. Multiple meta- analyses were performed on datasets from PGC-PTSD (PGC2) which includes data from the UK Biobank Study. For each PTSD dataset, meta-analyses interrogated populations that compared risks for male and female cohorts, as well as sub-populations of each sex independently. Results of these analyses demonstrated genotypic differentiation of overall PTSD risk based on sex and ancestry, as well as phenotypic differentiation noted in association with distinct risk loci based on those demographic differences, alone or in combinations of risk loci. These analyses highlight a polygenic risk factor involving variations in genes associated with the HPA axis, serotonergic and glutamatergic signaling, and inflammation. Additionally, GWAS findings emphasize the role of genetic-environment interactions, showing how trauma exposure interacts with genetic predispositions to affect PTSD susceptibility. These insights are paving the way for personalized approaches to PTSD prevention and treatment.
To begin to pave the way for personalized approaches to PTSD prevention and treatment, the FUMA (Functional Mapping and Annotation) pipeline was developed. FUMA is a bioinformatics tool used to analyze and interpret genetic loci from GWAS, including those linked to PTSD [16]. It enables the functional mapping of identified gene loci by integrating genomic data with existing biological knowledge to prioritize genes, pathways, and regulatory elements relevant to the disorder. Using the FUMA Pipeline approach, a total of twelve risk loci genes were identified and predicted to have both functional biological significance and psychiatric relevance for PTSD and related co-morbidities. European Ancestry risk loci genes included ZNF813, TMEM51-AS1,ZDHHC14, PARK2, KAZN, SH3RF3, and PODXL, wherein the latter two genes were identified through GWAS summary data for protein-coding genes. African Ancestry risk loci genes included LINC02571, TUC338, HLA-B, MIR5007 and LINC02335, but no risk loci genes were identified through GWAS summary data. The meta-analysis authors identified phenotypes among AFA subpopulations by using imaging data from the Grady Trauma Project based on changes in brain anatomy, and clinical psychophysiology [17]. The findings are presented in Table 1. This approach provides valuable insights into the genetic architecture of PTSD and helps identify potential therapeutic targets by linking genetic variation to biological function and clinical relevance.
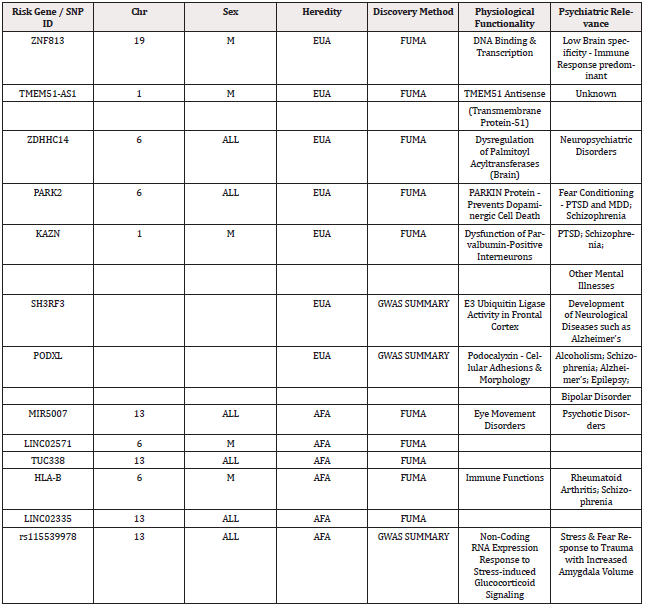
Table 1: Findings from the Meta-analysis of Psychiatric Genome Consortium (PTSD Group) - PGC-2 Dataset.
IG: intervention group; CG: control group
While the FUMA pipeline is a powerful tool for functional annotation of genetic loci, several challenges arise when leveraging its results for therapeutic strategies. First, PTSD is influenced by numerous genetic loci, each contributing small effects complicating pinpointing the most actionable targets for drug development. In addition, functional significance of variants identified by FUMA often requires extensive experimental validation, therefore translating annotated loci into mechanistic insights suitable for drug development is resource intensive. FUMA also relies on existing datasets, which may have gaps in coverage for certain tissue types, cell states, or rare variants relevant to PTSD, limiting the comprehensiveness of its annotations. Given the profound influence of environmental factors and epigenetic modifications, connecting genetic findings to dynamic, context-dependent biological processes. As such, even when plausible targets are identified, converting these into effective and safe drugs requires overcoming significant hurdles in preclinical and clinical phases, including issues with specificity and off-target effects.
Leveraging genetics and physiology for novel PTSD treatments involves targeting pathways and mechanisms implicated in the disorder and enhanced understanding of epigenetic modifications, such as trauma-induced changes in gene expression, opens avenues for interventions aimed at reversing symptoms and triggers of PTSD. Integrating genetic profiling with physiological biomarkers may enable personalized treatments that address individual vulnerabilities and symptom profiles, enhancing therapeutic outcomes in PTSD management.
A Novel, Artificial Intelligence Approach to Post-Traumatic Stress Disorder
PTSD treatments focus on harm reduction strategies but fail to target etiology of maladaptive behaviors. Such approaches ignore the balance of neurotransmitters in the reward pathways and fail to enhance target reward gene expression. An appropriate strategy to address the unmet need in PTSD treatment is leveraging a multiomics (genomics, transcriptomics, proteomics, and metabolomics) platform to identify molecular targets that are involved in dysregulated pathways.
Augmented intelligence (AI) algorithms integrated into a multiomic platform can be used to specifically address and “respond to” neurobiological pathways in PTSD prognostics and drug development to identify potential drug targets, optimize drug efficacy and minimize adverse effects, as “personalized” treatment approaches. This includes integration of computational modeling to simulate drug-receptor interactions and optimize drug binding affinity, selectivity, and potency, which in turn, can be used to design more effective drugs by predicting how drugs will interact with specific targets in the body.
AI promotes the capacity to utilize high technology programming to evaluate, learn, and reveal pharmaceutical-associated big data to design novel chemical entities (NCEs), by assimilating machine learning (ML) and deep learning (DL) on a highly integrated multiomics and mechanized AI platform. AI paradigms have established high relevance in the arena of drug design, vastly different from conventional approaches, in that AI circumvents the convoluted and established physio-chemical tenets. However, while ML and DL have achieved tremendous success in various domains, standard methods appear to have reached a plateau, as healthcare applications, data in healthcare applications are often highly imbalanced which slows down the democratization of AI. The capacity to layer multiple algorithms that integrate biostatistical approaches. employing imputed attributes and employing methods such as area under ROC curve (AUROC) and area under the Precision-Recall curve (AUPRC), is widely used for assessing the performance of a model, can optimize accuracy and break the bottleneck for further advancement. In turn, the algorithm can “learn” features from the input information in an automated fashion and is able to reorganize simple attributes into convoluted characteristics via multi-layer attribute extrication iteratively and dynamically via continuous interaction between computational predictions and experimental validations. This approach accelerates prognostication capacity and drug discovery by reducing the time and resources required to identify potential candidates with desired properties.
GATC Health Corporation (Irvine CA) is a science and technology company which utilizes Machine Learning algorithms, neural networks, and AI computational systems to efficiently generate diagnostic and prognostic evaluations based on genomic and other multiomic biomarkers. GATC utilizes a multi-omic approach which analyzes DNA, RNA, protein, metabolite, and lipid. Biomarkers use algorithms which mimic human systems biology. The platform, known as Multiomics Advanced Technology platform (MAT), imprints multi-omic data into model human systems biology algorithms which can accurately predict causal biomarkers of diagnostic and prognostic utility, and can also be used to generate therapeutics with high efficacy and minimal off-target effects. The MAT platform has been used to facilitate diagnosis of PTSD and other clinical conditions using these multiomic biomarkers and moreover, offers insights into prognosis and evaluation of new drug candidates leveraging both in-vitro and in-vivo models for in silico predictions and drug design. With this technology, GATC can pare down the set of leading candidate drugs for clinical testing to less than five likely successful compounds within months, instead of having to test over 5,000 compounds over four to six years. This unique process can dramatically reduce the time and resources required to identify lead compounds thereby enhancing pharmaceutical quality and improving effectiveness and safety in humans.
Proof in Concept
The GATC MAT approach initially created a family of over eighty lead compounds designed specifically as a strategy for mitigating opioid use disorder (OUD), a comorbidity in nearly 20% of veterans with PTSD [17]. The multiomic comprehensive datasets were subsequently analyzed by the GATC Heath team with algorithmic processes which apply iterative ML methods to discover and validate in silico NCEs designed to increase an addicted patients’ feeling of wellbeing while improving brain function; this is accomplished by remodeling the circuits involved with addictive behavior and creating a cycle for continued improvement. The receptor classes affected by the compounds are expected to enable the patient to rapidly feel the benefits of the drug, promoting improvement in brain function with continued treatment and resulting in an increased feeling of wellbeing. These functional parameters were programmed into the algorithms by specifically targeting receptors well-established as active in addicted brains including neuron-derived C-C motif chemokine ligand 2 (CCL2), brain-derived neurotrophic factor (BDNF), and the structurally and functionally related activator proteins (JUNa, FOSb). These receptors are associated with inflammation, translocation, neurite outreach and neurohormone balance, physiologically related pathways for PTSD.
Biochemical models suggest that balancing of neurohormones by concurrently targeting multiple receptors typically perturbed in OUD, will assist in “weaning” the brain off desiring high levels of dopamine. The consequent re-balancing promotes homeostatic synthesis and release of enhanced mood promoting hormones including oxytocin, serotonin, and endorphins. The receptor-drug interaction strategy was not designed to artificially suppress or even lower dopamine levels, but rather to increase dopamine in the nucleus accumbens (NA) without creating euphoria in the patient. The NA is thought to be the neural interface between motivation and action, playing a key role on feeding, sexual, reward, stress-related, addiction and drug self-administration behaviors (18). To accomplish such hormone, rebalance, the algorithms were also designed to generate compounds which stimulate the tropomyosin receptor kinase B (TKRB), 5-hydroxytryptamine (5HT, serotonin), oxytocin (OXT) and triphosphate-dependent metabolic processes while exhibiting partial selectivity toward the prefrontal cortex and the NA12. TrkB is a protein that in humans is encoded by the NTRK2 gene. TrkB is a receptor for BDNF12,13. Serotonin, also known as (5- HT), is a monoamine neurotransmitter. It also acts as a hormone. OXT is released in response to different physiological stimuli, playing a key role in reaction to stress and in reducing proinflammatory responses (19) . The strategy relies on intermittent stimulation of these pathways and as an in situ methodological approach for the addict’s brain to undergo limbic remodeling while also maintaining sufficient levels of dopamine to ameliorate the symptoms of withdrawal and craving [20]. The NCEs have been chemically)-synthesized ready for the comprehensive pre-clinical evaluation phase of drug development proposed herein. The salient characteristics of these NCEs: 1) are not expected to pose any risk for abuse, rather provide incentive for use to increase the feeling of comfort not euphoria; 2) are expected to remodel the limbic system, addressing the root problem of addiction by improving the addicted brain neuroplasticity; and 3) will all exhibit low toxicity profiles, therefore safe for human use. The panel of lead molecules generated by the GATC Health drug discovery algorithm to treat pain-management related addiction began with in vitro validation and has been initiated in pre-clinical animal studies. In parallel, data sources from comprehensive biological domains continue to accumulate, providing significant opportunities to integrate into MAT platform-based models in PTSD.
Recently, new genomic approaches in the use of GATC MAT platform are helping to identify alternative biomarkers and processes aiding in PTSD predictive diagnostics, as well as targets for mitigation of PTSD symptoms through pharmacotherapy. Independent neuroscientists working with PTSD have also confirmed that the rACC plays a pivotal role in the development of PTSD symptoms in affected individuals [21]. Cognition, emotional processing, and decision-making are all managed to a great degree in the rACC and are modulated by four groups of neurotransmitters: Glutamic acid (GLU), dopamine (DA), serotonin (5-HT), and Gamma-aminobutyric acid (GABA). Depending on whether they are stimulatory, inhibitory, or neuromodulators for various groups of neurons, they have different effects on the reactivity and firing rate at synapses in the rACC, thereby potentially altering the symptom manifestations accompanying psychiatric disorders, such as PTSD. These neuromodulators and the neurons that they affect, represent prospective targets for pharmacotherapy of behavioral health disorders. Modulation of these neural processing areas in the rACC can lead to improvements in emotional regulation, regulation of traumatic memories, and other symptoms, thereby collectively eliciting more successful PTSD treatment outcomes.
Building upon the insights gained from previous research, AI allows a deeper understanding and predicting PTSD, identifying biomarkers associated with risk and subsequently overcoming the gap between discovery and translating efforts to expedite progress toward clinical trials design. This novel strategy applied a complex integration of genomic, transcriptomic, proteomic and metabolomic biomarker are underway to enhance the MAT platform’s multiomics framework by integrating the extant “-omics” readouts and resources, generating new supplemental data in discovery areas lacking sufficient depth, especially PTSD. Simultaneously, an integrated catalog of results and biological targets will be created. Considerable evidence linking PTSD symptomology to the rostral Anterior Cingulate Cortex (rACC) and neuroinflammatory processes highlights the opportunities to target these areas.
Promising drug candidates for PTSD identified by MAT platform are currently being evaluated within the context of regulatory science pathways. The inclusion of exposure variables adds to the predictive power of AI. Classification-based ML may be useful in predicting risk of future PTSD in populations with anticipated trauma exposure. As more data become available, including additional molecular, environmental, and psychosocial factors in these scores may enhance their accuracy in predicting PTSD and, relatedly, improve their performance in independent cohorts, GATC will include testing via in-vitro and in-vivo pre-clinical studies.
GATC’s research process started by utilizing the power of the MAT platform to efficiently discover compounds targeted at PTSD mitigation and treatment. The Company scientists configured experiments on the MAT platform to exclude candidate drugs that would have toxic, side-effect prone, or material off-target properties. The initial library of PTSD compound leads was produced by the platform from a curated set of multi-omics data collected from a variety of anonymized PTSD patient databases, which were used as inputs for the Platform’s experimental iterations. Analysis of this library shows these compounds possess the necessary pharmacologic properties to improve mental resilience and acuity of cognition. The novel candidate PTSD drugs were then synthesized in preparation for pre-clinical evaluation studies. Key to these discoveries was the combination of uncovering the necessary neuro-modulation enhancements through detailed anatomic and physiological analysis of the post-mortem human brain tissue from patients with PTSD and using in-silico advanced neuronal modeling on the GATC platform. To minimize the impact of false inflation of linkages of causal gene loci, a multifaceted methodology that stands out in its effectiveness and precision was utilized. Central to the approach is the utilization of large, well-powered datasets, which are instrumental in detecting true causal associations while minimizing false positives. This is especially pertinent in disorders like PTSD, where genetic influences are often subtle and easily masked. These studies will assess the specificity, safety, and toxicity of these PTSD mitigation drug candidates and ensure low toxicity and the required safety for use in humans, while increasing the opportunity for successful investigational new drug (IND) opening “First-in-Human” (FIH) clinical trial.
Ideally, an AI MAT platform such as that developed by GATC provides an opportunity to simulate human biology and physiology to predict an individual’s risk of developing PTSD. Further, under specific conditions, including the stresses of deployments in active combat environments, genomic and proteomic biomarkers could evaluate predisposition for developing PTSD symptoms. In addition, the technology helps guide the development of unique therapeutics, particularly novel, FDA approved drugs, as well as natural treatments, for the mitigation of PTSD. Integrating technology with clinical recommendations would facilitate appropriate medication dosing and forecasting potential off-target effects, for individuals diagnosed with, or susceptible to, PTSD. Further, long-term application addresses the challenge of identifying patient-appropriate subsets of novel PTSD medications, that when combined with appropriate psychotherapeutic techniques, such as Cognitive Behavioral Therapy (CBT) and/or Immersion Therapy (IT), are likely to achieve a better patient outcome, than psychotherapy alone, or in combination with older medications, such as antidepressants, that have traditionally been prescribed for clinical management of PTSD.
Clinical And Field Applications
The future holds the possibility of testing individual military personnel to capture both their genetic makeup, and active genomic & proteomic expression patterns using standardized saliva and blood samples. Using such data, GATC Health’s MAT™ platform can evaluate individual service personnel and provide a stratified predisposition to stress-induced incidence of PTSD. This analysis will simultaneously assess their potential for clinical benefit from prophylactic medication and other measures, such as psychotherapy. The major advancement is a prophylactic approach in which MAT-platform-designed, “Smart Drugs” can potentially be effective to 1) stimulating neuroplasticity to enhance emotional processing resilience; 2) decrease the perceived magnitude of emotional trauma by inducing a small “separation” effect between perception and emotion, thus increasing mental acuity and objective operational judgement during high stress engagements; and 3) enhance neural signaling and emotional processing during psychotherapy for PTSD symptoms may reduce adverse events occurring in-between treatment sessions, by mitigating the risk of aggression by personnel with PTSD, towards other Veterans and/or military personnel. In the context of military readiness and pre-habilitation, the goal is to enable recruits to receive, if warranted, pharmacological prophylaxis directed towards enhancing behavioral response, and increase the cognitive acuity required from warfighters during high-stress engagements, without compromising their emotional stability and well-being. Thus, the risk of warfighters manifesting PTSD symptoms will be reduced. A two-part approach beginning with evaluation of incoming recruits to understand their genetic makeup (genotype), as well as genetic expression (phenotype). Using this data, their relative need for prophylactic measures, and proclivity to stress-induced incidence of PTSD, can be identified such that pharmacological prophylaxis can then be directed towards patient-centric utilization.
Challenges
Despite recent advances, significant gaps remain in understanding how PTSD and related comorbidities develop across the lifespan. Much of the research on stress in veterans has focused on broad categories of adversity and limited protective factors, overlooking specific stress dimensions, timing of exposure, and individual variability. These gaps limit our ability to understand how distinct types of early adversity, such as threat or deprivation, uniquely shape adult stress responses and health outcomes.
The integration of intersectionality via AI and ML has enormous potential to illuminate how intersecting stressors influence biology and health. Incorporating intersectionality could enhance precision medicine and address disparities in health outcomes. Stress-related pathologies, likely-arise from multisystem dysregulation, necessitating comprehensive approaches that assess interactions among genetic, environmental, and biological factors are needed to advance our understanding and treatment options.
Summary
Building on current developmental theory and research, the MAT platform, developed by GATC wields the power to stimulate neuroplasticity, which enhances emotional processing resilience and concomitantly decreases the perceived magnitude of emotional insult, by creating a small uncoupling between perception and emotion, and as a result, can increase cognitive acuity during high stress engagements. The multi-omics approach is well-positioned to produce major breakthroughs in the understanding of the development of PTSD pathology. By combining methodological approaches as described herein, physicians can deliver targeted doses to enhance desirable emotional responses and mental acuity, without compromising emotional well-being. The overarching aim of these types of platforms is to evaluate new PTSD mitigation drug candidates which have been identified by a novel AI-based end-to-end drug development platform to address the increasingly expanding unmet needs in PTSD treatment.
Acknowledgments
The authors acknowledge the support of GATC Health and UC Irvine for their support of this project.
References
- Health NioM (2024) Post-Traumatic Stress Disorder (PTSD).
- Brady KT, Killeen TK, Brewerton T and Lucerini S (2000) Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry 61(Suppl 7): 22-32.
- Burback L, Bremault Phillips S, Nijdam MJ, McFarlane A and Vermetten E (2024) Treatment of Posttraumatic Stress Disorder: A State-of-the-art Review. Curr Neuropharmacol 22(4): 557-635.
- Affairs USDoV (2014) Study explores reasons why Veterans seek-or don't seek-PTSD care.
- Back SE, Killeen T, Badour CL, Flanagan JC, Allan NP, et al (2019) Concurrent treatment of substance use disorders and PTSD using prolonged exposure: A randomized clinical trial in military veterans. Addict Behav 90: 369-77.
- Richardson LK, Frueh BC and Acierno R (2010) Prevalence Estimates of Combat-Related PTSD: A Critical Review. Aust N Z J Psychiatry 44(1) :4-19.
- (2024) Affairs USDoV. Combat Exposure.
- Schein J, Houle C, Urganus A, Cloutier M, Patterson-Lomba O, et al (2021) Prevalence of post-traumatic stress disorder in the United States: a systematic literature review. Curr Med Res Opin 37(12): 2151-2161.
- Qureshi KL, Upthegrove R, Toman E, Sawlani V, Davies DJ, et al (2019) Post-traumatic stress disorder in UK civilians with traumatic brain injury: an observational study of TBI clinic attendees to estimate PTSD prevalence and its relationship with radiological markers of brain injury severity. BMJ Open 9(2): e021675.
- Bowers ME and Yehuda R (2017) Neuroendocrinology of Posttraumatic Stress Disorder: Focus on the HPA Axis In: G F, editor. Stress: Neuroendocrinology and Neurobiology. San Diego: Academic Press 6123: 165-172.
- Vermetten E and Bremner JD (2002) Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depress Anxiety 16(1): 14-38.
- Gelernter J, Sun N, Polimanti R, Pietrzak R, Levey DF, et al (2019) Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat Neurosci 22(9): 1394-1401.
- Swaab DF and Bao AM (2020) Sex differences in stress-related disorders: Major depressive disorder, bipolar disorder, and posttraumatic stress disorder. Handb Clin Neurol 175: 335-358.
- Stein MB, Levey DF, Cheng Z, Wendt FR, Harrington K, et al (2021) Genome-wide association analyses of post-traumatic stress disorder and its symptom subdomains in the Million Veteran Program. Nat Genet 53(2): 174-184.
- Tseilikman VE, Tseilikman OB, Pashkov AA, Ivleva IS, Karpenko MN, et al (2022) Mechanisms of Susceptibility and Resilience to PTSD: Role of Dopamine Metabolism and BDNF Expression in the Hippocampus. Int J Mol Sci 23(23): 14575.
- Nievergelt CM, Maihofer AX, Atkinson EG, Chen CY, Choi KW, et al (2024) Genome-wide association analyses identify 95 risk loci and provide insights into the neurobiology of post-traumatic stress disorder. Nat Genet 56(5): 792-808.
- Seligowski AV, Duffy LA, Merker JB, Michopoulos V, Gillespie CF, et al (2021) The renin-angiotensin system in PTSD: a replication and extension. Neuropsychopharmacology 46(4): 750-755.
- Xu Y, Lin Y, Yu M and Zhou K (2024) The nucleus accumbens in reward and aversion processing: insights and implications. Front Behav Neurosci 18: 1-22.
- Panaro MA, Benameur T and Porro C (2020) Hypothalamic Neuropeptide Brain Protection: Focus on Oxytocin. J Clin Med 9(5): 153.
- (2024) Affairs USDoV PTSD and Substance Abuse in Veterans.
- (2016) (US) SAaMHSA. Chapter 2. The Neurobiology of Substance Use, Misuse, and Addiction Washington DC: US Department of Health and Human Services.
- Niazi SK (2024) Placebo Effects: Neurological Mechanisms Inducing Physiological, Organic, and Belief Responses-A Prospective Analysis. Healthcare (Basel) 12(22): 2314.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.