Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Exploring the Mechanism of Ganoderma lucidum in Treating Hyperlipidemia Based on Network Pharmacology and Molecular Docking Technology
*Corresponding author: Zheng-Lian Xue, College of Biology and Food Engineering, Anhui Polytechnic University, Wuhu Green Food Industrial Research Institute Co., LTD, 241000, Wuhu, China
Received: March 04, 2025; Published: March 10, 2025
DOI: 10.34297/AJBSR.2025.26.003413
Abstract
Network pharmacology and molecular docking methods were used to study the mechanism of Ganoderma lucidum in treating hyperlipidemia and to provide the basis for basic research and clinical research. Active ingredients of Ganoderma lucidum were screened by the TCMSP database and hyperlipidemia-related targets were screened by the Genecards, DisGeNet, and OMIM databases. The protein interaction network and active ingredient-key target pathway were constructed using CytoScape 3.9.1 software. Gene function and pathway enrichment analysis were carried out with the help of the DAVID database, and molecular docking of crucial active ingredients and core targets was performed with AutoDock Vina software to screen potential targets and related signaling pathways of Ganoderma lucidum active ingredients. PPARA, TNF, ESR1, and EGFR were the critical targets of Ganoderma lucidum in treating hyperlipidemia. The key signaling pathways mainly involve the PPAR, insulin resistance, and TNF signaling pathways. The molecular docking results showed that the binding energy of the 7 main active ingredients had good binding with EGFR and CYP3A4, and the binding energies were all less than -6.5kcal·mol-1. The active ingredients of Ganoderma lucidum can treat hyperlipidemia by acting on multiple targets through multiple pathways, providing a specific scientific basis for basic experimental research and clinical application.
Keywords: Ganoderma lucidum; Hyperlipemia; Network pharmacology; Molecular docking; Action mechanism
Introduction
Hyperlipidemia (HLP) arises from disruptions in blood lipid metabolism, typically evidenced by a decline in high-density lipoprotein cholesterol levels or elevations in total cholesterol, triglyceride, and low-density lipoprotein cholesterol concentrations [1]. HLP is not merely a precipitating factor for obesity and type 2 diabetes but also intimately linked to various metabolic disorders, including elevated blood lipid levels, hepatic cirrhosis, and kidney failure diseases [2]. Currently, the primary therapeutic options for managing HLP primarily encompass Western medications like simvastatin, fenofibrate, and clofibrate. While this medicine effectively addresses the condition, their efficacy is often focused on a single target, limiting their comprehensive impact on multiple aspects of HLP. The prolonged administration of these medications frequently entails unwanted consequences, including indigestion and impairment of Liver and kidney functions, among others, with a heightened risk of relapse upon cessation of treatment [3]. As a result, the exploration of plant-based medications has garnered significant attention.
Ganoderma lucidum was first published in Shennong Herbal Classics [4]. This herb boasts a medicinal history spanning over two millennia in China and is currently listed in the Pharmacopoeia of the People’s Republic of China (Volume I) for its therapeutic applications [5]. Ganoderma lucidum is a medicinal and edible homology fungus of Ganoderma lucidum family. It has a sweet taste, smooth sex, and heart, lung, liver, and kidney channels. It also has functions such as relieving cough, calming asthma, invigorating qi, and calming nerves. Since ancient times, Ganoderma lucidum has been a good medicine for promoting health, keeping youth and vitality alive forever, and prolonging life. Ganoderma lucidum has a range of bioactive components, including polysaccharides, triterpenoids, sterols, proteins, amino acids and nucleosides [6]. Modern scientific research has found that Ganoderma lucidum has pharmacological functions such as anti-oxidation, lowering blood sugar, lowering blood lipids, anti-tumour and regulating immunity, and has been widely used in drugs, health products and cosmetics [6]. According to Zhu et al., polysaccharides can reduce serum cholesterol and increase HDL cholesterol levels and insulin sensitivity from Ganoderma lucidum, suggesting that Ganoderma lucidum has a positive effect on treating hyperlipidemia [7]. Until now, few studies have been on the anti-HLP effect of Ganoderma lucidum. In addition, due to the diversity and structural complexity of Ganoderma lucidum active ingredients, obtaining an anti-HLP target and specific mechanism network is challenging.
Network pharmacology is a concept first proposed by Hop-kins [8]. Network pharmacology, situated within the realm of systems biology and pharmacology, revolves around the construction of intricate interaction networks that intertwine compounds, genes, protein targets, and diseases, offering a holistic view of their interconnectedness [9]. Network pharmacology has evolved into a comprehensive “disease-gene-target-drug” network model, enabling a multi-faceted understanding and evaluation of the molecular mechanisms underlying drug action [10]. This study aims first to identify the potential components, targets, and molecular pathways involved in Ganoderma lucidum’s anti-HLP effects through the “active ingredient-target-signaling pathway” network pharmacology approach. Subsequently, molecular docking technology will be used to validate the underlying mechanisms of Ganoderma lucidum’s anti- HLP action. This approach provides methodologies and theoretical foundations for elucidating Ganoderma lucidum’s mechanism of action.
Materials and Methods
Screening of Chemical Constituents and Corresponding Targets of Ganoderma Lucidum
To identify potential active components from Ganoderma lucidum, a search was conducted in the Traditional Chinese Medicine Systems Pharmacology (TCMSP, https://tcmspw.com/index. php) database using “Ganoderma lucidum” as the keyword. The screening criteria included an Oral Bioavailability (OB) threshold of ≥30%, a Drug-Likeness (DL) score of ≥0.18, a relative molecular weight of ≤500, a maximum of 5 hydrogen bond donors, and a maximum of 10 hydrogen bond receptors. This rigorous approach aimed to narrow down the selection to the most promising candidates [11-13]. Utilizing the PubChem database (https://pubchem. ncbi.nlm.nih.gov/), the 2D structures of the identified compounds were downloaded and subsequently imported into the Swiss Target Prediction platform (http://www.swisstargetprediction.ch/). By specifying “Homo sapiens” as the target organism and initiating the prediction process, the corresponding targets were forecasted. Targets with a predicted probability of zero were excluded, and the remaining data was consolidated to eliminate duplicates [14].
Screening of Hyperlipidemia Targets
To further analyze the potential therapeutic targets for HLP, searches were conducted in the GeneCards database (https://www. genecards.org/) [15] using “Hyperlipidemia” or “HLP” as keywords, with a “relevance score” of≥7.0 as the filtering criterion. Additionally, the DisGeNET database (http://www.disgenet.org/) [16] and the OMIM database (Online Mendelian Inheritance in Man, https:// omim.org/) [17] were also queried using the exact keywords to gather comprehensive information on disease-associated genes. As for the OMIM datasets, all targets related to HLP were involved. Then, they combined the targets of the three databases to get the final target of HLP by removing duplicate genes [18].
Screening of Effective Components and Common Disease Targets
The species was set as Human through the UniProt (https:// www.uniprot.org/) database, and the gene name of the target protein was determined. Then Venny diagram tool (https://bioinfogp. cnb.csic.es/tools/venny/index.html) was used to intersect the active ingredients in Ganoderma lucidum and the disease targets, which were the potential targets of Ganoderma lucidum in the treatment of HLP.
Construction of “Active Compound-Target” Network
Utilizing the Cytoscape 3.9.1 platform (http://cytoscape.org/), we constructed an active compound-target network featuring candidate active compounds and their potential targets derived from Ganoderma lucidum [14]. The core of this network comprised nodes representing the active components and targets, while the connections between these nodes illustrated their interdependent biological interactions [19].
The Construction of Protein-Protein Interaction (PPI) Networks
The intersecting targets were uploaded to the STRING database (https://string-db.org/) for PPI (protein-protein interaction) network analysis. The species was specified as “Homo Sapiens,” and a minimum required interaction score of >0.4 was applied. All other settings were left at their default values to identify the crucial PPI targets [20]. The PPI network map was exported in “TSV” format and subsequently imported into the Cytoscape software (version 3.2.1, accessible at http://cytoscape.org/) for visualization, creating a comprehensive and interactive network representation. The visual representation of the network diagram was customized, with node sizes and colors reflecting the degree and betweenness centrality, respectively. Edges were used to illustrate connections between active ingredients and their targets, as well as interactions between targets and biological pathways. Ultimately, a clear understanding of the relationships among the active ingredients, their potential protein targets, and the associated pathways was achieved.
Analysis of Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Pathway
The intersection targets were analyzed using the DAVID database (https://david.ncifcrf.gov/) with “Homo sapiens” as the species, applying a statistical significance threshold of P<0.05. This process involved performing Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis [21]. To visualize the enrichment results, the bioinformatics online platform (http://www.bioinformatics.com.cn/) was utilized, with statistical significance determined by a P-value threshold of <0.05 [22].
Compound-Target-Pathway for Ganoderma Lucidum as a Treatment for Hyperlipidemia Network Construction
Utilizing the active compounds identified in Ganoderma lucidum, a “Compound-Target-Pathway (C-T-P)” network aimed at treating HLP was constructed with the aid of Cytoscape 3.9.1 software. The KEGG pathway analysis revealed intersections between HLP-related target genes and the active compounds present in Ganoderma lucidum. This information was used to construct a network where each compound, target, or pathway was represented by a node, and their interactions were depicted by edges. Additionally, the Network Analyzer plugin in Cytoscape 3.9.1 was employed to quantify the degree of node interaction by analyzing the number of connecting edges [22].
Molecular Docking
The key active ingredients were used as ligands, and the key targets were used as receptors. To acquire the 3D structure of the ligand, the PubChem database was utilized. For the target proteins, the RCSB PDB database (https://www.rcsb.org) was searched, and only those proteins with a crystal resolution of less than 2 Å were selected for their 3D conformations. The receptor macromolecule was prepared using PyMOL software by removing water molecules and proto-ligand small molecules, followed by saving in PDB format. AutoDock software was then employed to refine the protein structure through dehydration, hydrogenation, charge calculation, and assignment of atom types, ultimately saving it in PDBQT format. Small molecule ligands were optimized using Chem3D to achieve minimal free energy, and molecular docking was carried out with AutoDock Tools-1.5.6. The docking results were visualized using PyMOL for analysis.
Results
Screening of Active Components
Utilizing the TCMSP database, the chemical components of Ganoderma lucidum were screened based on criteria of an Oral Bioavailability (OB) greater than 30% and a Drug-Likeness (DL) score exceeding 0.18, resulting in the identification of seven active components. 274 effective targets were obtained after the removal of duplicates.
Screening of Hyperlipidemia-Related Targets and Acquisition of Intersection Targets
The HLP target results retrieved from the Genecard, OMIM, and Drugbank databases were combined, and the duplicated disease genes were discarded to obtain 1115 HLP-related gene-disease targets. The results were obtained by Venn 2.1.0. As shown in Fig.1, blue represents the relevant targets of Ganoderma lucidum compounds (274) and yellow represents the relevant targets of HLP (1115). Eventually, 68 overlapping components and disease targets were intersected for further analysis, which were the targets of Ganoderma lucidum treating HLP (Figure1).
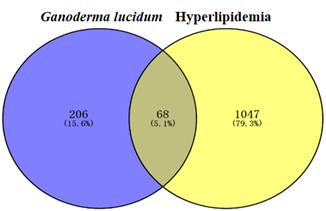
Figure 1: Venn diagram of the potential targets of Ganoderma lucidum in the treatment of hyperlipidemia.
Compound-Target (C-T) Network Analysis
The “Component-target” network of Ganoderma lucidum compounds was assembled through Cytoscape (version 3.9.1). Among them, the dark red square nodes described the potential active compound of the Ganoderma lucidum compounds, the dull yellow circle nodes described the action targets, and each edge described the action relationship from compounds to targets. This network comprised a total of 281 nodes, including 7 dedicated to bioactive compounds and 274 representing target nodes, which interconnected by 705 edges (Figure 2). The node degree value quantifies the number of edges attached to a node, where a higher value signifies a greater number of connected compounds or targets, thereby increasing the likelihood of being a pivotal compound or target (Table 1).
PPI Network Analysis
The STRING 11.0 database was employed to analyze the 68 potential targets of Ganoderma lucidum’s active ingredients in the context of treating HLP. As shown in Figure 3, the protein interaction topology contained 68 nodes and 502 edges. The importance of the target in the network can be judged by the area and color depth of the nodes and the node degree value. The mean point degree value was 14.8, the average clustering coefficient was 0.629, and the P<1.0e×10-16, indicating that the PPI network had significant statistical significance. These 68 targets could be crucial factors contributing to the therapeutic mechanism of Ganoderma lucidum in managing HLP.
The PPI results were taken as TSV format was analyzed using Cytoscape 3.9.1 software for visualization and topology analysis. The degree value reflected the extent of node connection, which indicated the degree of correlation. When the number of edges consistent with the target of a compound was significant, it meant that the compound greatly impacted the treatment. Betweenness values indicate the ability of a compound to communicate between all the shortest paths [23]. The node embodies the pivotal target, whereas the edge depicts protein-protein interactions. A heightened degree of network connectivity signifies stronger relationships among proteins, with a corresponding increase in node degree value resulting in a larger protein node representation. The results were depicted in Figure 4 and Table 2, and the essential targets were as follows: TNF (47), PPARG (43), PPARA (36), TP53 (31), EGFR (28), HIF1A (28), ESR1 (28), and CYP3A4 (27), which may play key regulatory roles in protein interaction networks (Table 2). Based on these results, we believe these could be crucial target genes in developing hyperlipemia.
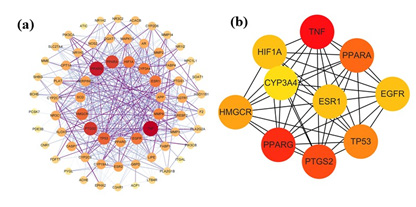
Figure 4: PPI network of core targets for Ganoderma lucidum against hyperlipidemia.
a) The PPI network map optimized by Cytoscape
b) The top 10 core targets of PPI network map
GO Function and KEGG Pathway Enrichment Analysis
To further explore the mechanism of action of Ganoderma lucidum against hyperlipemia, the key target genes were analyzed by GO and KEGG signaling pathways. DAVID works for online bioinformatics analysis databases to help identify and capture large amounts of information about gene or protein function [24]. The David database was searched, and 68 targets were used for biological function analysis. GO enrichment yielded 350 entries (P<0.01), of which the enrichment of biological process (BP, 254 records), molecular function (MF, 57 recordings), and cellular component (CC, 31 records). The P-value of the items was negatively correlated with the enrichment degree. The items were arranged in ascending order according to the P-value and count. The top 10 entries from each of the BP, MF, and CC categories were selected to create bar charts for visualization (Figure 5). As shown in Figure 5, the BP category results revealed that the positive regulation of miRNA transcription, cholesterol metabolism process and negative regulation of miRNA transcription were the important biological processes in the treatment of hyperlipemia; CC category was primarily engaged in the endoplasmic reticulum, chromatin and endoplasmic reticulum; and MF category was mostly implicated in nuclear receptor activity, steroid binding and enzyme binding.
The potential targets were enriched by KEGG pathway analysis, and 102 signaling pathways were obtained. In ascending order of P-value, select the first 20 paths. The more genes involved in the pathway, the larger the bubble; The greater the statistical significance, the darker the fill color from green to red. The 20 pathways were depicted in Figure 6, the core pathways were mainly involved in Endocrine resistance (Count=10, P=2.38E-8)、PPAR signaling pathway (Count=9, P=4.77E-8)、 Insulin resistance (Count=10, P=5.55E-8) and regulation of lipolysis in adipocytes (Count=7, P=3.14E-6). The findings revealed that the potential targets of Ganoderma lucidum compounds in treating HLP were widespread across various pathways, working in concert to modulate multiple structures and functions.
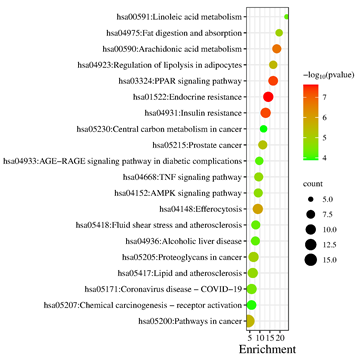
Figure 6: KEGG pathway enrichment analysis of potential targets of Ganoderma lucidum against hyperlipidemia.
Construction of Compound-Active Ingredients-Potential Target- Pathway Network
The Cytoscape 3.9.1 software was used to construct the interaction network diagram of the “Chinese Medicine-active ingredients- potential target-pathway” of Ganoderma lucidum in treating hyperlipemia, in which the node size reflected the intensity. As shown in Figure 7, the network contained 95 nodes (blue for targets, yellow for compounds, and green for pathways) and 377 edges, which showed that the targets of Ganoderma lucidum chemical components were distributed in different metabolic pathways, with multiple molecules acting on the same pathway and one molecule acting on multiple pathways. According to this network, TNF、ESR1 、TP53、EGFR can be considered as the hub gene, as it were enriched in almost all enriched pathways, PPAR signaling pathway、 Insulin resistance lipids and atherosclerosis and regulation of lipolysis in adipocytes.
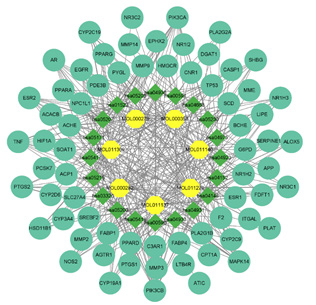
Figure 7: “drug-core ingredient-core target-disease” interactive network potential targets of Ganoderma lucidum in treating hyperlipemia.
Molecular Docking Verification
The seven active compounds identified from Ganoderma lucidum were individually docked with the top hub genes. Binding energies less than 0 kcal·mol-1 were indicative of spontaneous molecular protein binding, with lower values signifying greater stability in molecular conformation. Binding energies below -5 kcal·mol-1 were considered to demonstrate a stronger bonding force [25, 26]. The active compound with the lowest binding energy to TP53, TNF, PPARG, PPARA, HIF1A, ESR1, EGFR and CYP3A4 were Cerevisterol (-6.75 kcal·mol-1), Campesta-7,22E-dien-3β-ol (-7.65kcal·mol-1), Cerevisterol (-6.93kcal·mol-1), Valeric acid (-6.59 kcal·mol-1), Valeric acid (-7.42 kcal·mol-1), ErgoSTA-7,22E-dien-3β-ol (-5.57kcal·mol-1), Campesta-7,22E-dien-3β-ol (-8.88 kcal·mol-1) and ErgoSTA-7,22Edien- 3β-ol (-8.68kcal·mol-1) (Figure 8).
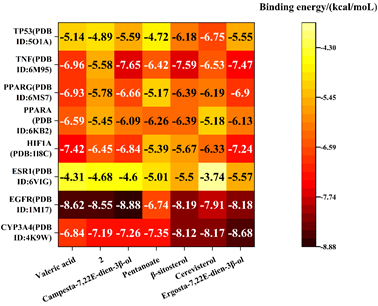
Note*: 2 was 5α-Lanosta-7,9(11),24-triene-15α,26-dihydroxy-3-one.
Figure 8: Heat map of molecular docking between the main active components and key targets of Ganoderma lucidum in treating hyperlipemia.
The results of the visual simulation analysis were shown in Figure 9. The three-dimensional visualization of the ligand-receptor complex showed that the compounds could stabilize the target proteins’ active pockets, respectively. The findings showed that the binding from core components to key targets was mainly hydrophobic, and hydrogen bonds and salt bridges also played a role in molecular binding (Table 3). It was speculated that the above active ingredients were the critical pharmacodynamic ingredients of Ganoderma lucidum in treating hyperlipemia.
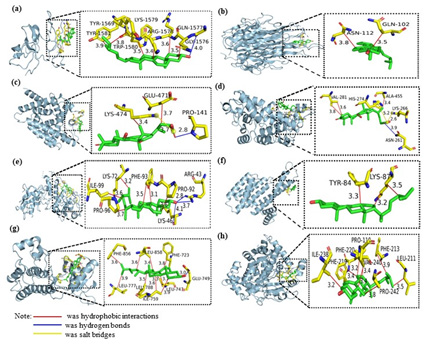
Note*: the yellow stick-like part was the stick-like structure of amino acid residues connected to the core targets, and the green stick-like part was the active compounds of Ganoderma lucidum. The number indicated the length of the bond of the compound.
Figure 9: The 3D visual displays of ingredient-protein molecular docking mode
a) Ergosta-7,22E-dien-3β-ol and TP53
b) Campesta-7,22E-dien-3β-ol and TNF
c) Valeric acid and PPARG
d) Valeric and PPARA
e) Pentanoate and HIF1A
f) Ergosta-7,22E-dien-3β-ol and ESR1
g) Campesta-7,22E-dien-3β-ol and EGFR
h) Ergosta-7,22E-dien-3β-ol and CYP3A4
Discussion
With the change of modern diet structure, the incidence of HLP has increased significantly, and HLP has become a significant public health problem [27]. In this research, we employed network pharmacology as a means of analyzing and found that the seven antihypertensive- active compounds in Ganoderma lucidum included campesta-7,22E-dien-3β-ol, cerevisterol, β-sitosterol, pentanoate, and other key active ingredients. Studies have confirmed that compounds like β-sitosterol, belonging to the phytosterol family, exhibit capabilities in enhancing cholesterol metabolism, mitigating oxidative stress, and diminishing lipid build-up in hepatocytes subjected to high fatty acid concentrations [28].
By conducting an in-depth examination of the C-T network and PPI network, PPARG, TNF, EGFR, ESR1, and other targets were considered the critical targets of Ganoderma lucidum in the treatment of hyperlipemia. The formation of adipocytes is a complex process in which the regulation of gene expression is crucial [29]. Studies have found a variety of positive transcription factors and reverse transcription factors that affect the differentiation function of adipocytes, among which PPARG is a factor that cannot be underestimated in the process of adipocyte formation [30]. In addition to regulating adipocyte differentiation, PPARG can also regulate adipocyte metabolism and insulin sensitivity of mature adipocytes, playing an essential role in glycolipid metabolism [31,32]. PPARG potentiated the adipose process, and enhanced the process of adipogenesis [33]. Upregulation of PPARG can affect genes involved in fatty acid metabolism and triacylglycerol storage to maintain adipocyte phenotypes, affecting blood lipids [34]. Therefore, PPARG was closely related to fat formation. ESR1 mediated the expression of the SCL2A4 gene to affect the standard transformation of fat to regulate blood glucose metabolism disorders [35]. Inflammation is also closely related to HLP, which can accelerate fat accumulation in liver cells. A considerable accumulation of fat can aggravate the inflammatory response and activate and enhance the expression of inflammatory factors. As an inflammatory element, TNF, a critical cytokine, was predominantly secreted by activated macrophages and natural killer cells, in which Tumor necrosis factor-α played a significant role. TNF family protein levels have been an essential indicator in HLP-related diseases [36]. EGFR is involved in many human diseases and is central to regulating the survival, proliferation, migration, and differentiation of various tissues. Research has revealed EGFR’s link to metabolic disturbances, where in it mitigates fat accumulation and hepatic cellular injury through diminishing cholesterol biosynthesis and fostering fatty acid oxidation. Consequently, EGFR emerges as a promising therapeutic target for addressing dyslipidemia [37]. ESR1 is one of the main types of estrogen receptor, and ESR1 gene polymorphism plays a crucial role in lipid metabolism [38]. CYP3A4 is involved in the bio-transformation and transportation of bile acid, as well as the metabolism of cholesterol [39].
The KEGG pathway analysis highlighted the prevalence of several key pathways, including insulin resistance, TNF signaling, PPAR signaling, and endocrine resistance pathways, as the primary ones among the observed enrichments. Insulin resistance is closely related to HLP, and patients with HLP are prone to insulin resistance. At the same time, insulin resistance makes insulin target cells insensitive to insulin, and target cells cannot take up plasma glucose, resulting in increased plasma glucose levels [40]. The body needs to absorb excessive glucose in plasma to synthesize fat and store it in target cells. Maintain the balance of glucose metabolism, causing HLP. TNF-a was one of the secretion products of adipocytes. It could promote the apoptosis of precursor adipocytes and adipocytes, inhibit the differentiation of precursor adipocytes, and inhibit the synthesis of adipocytes [41]. Furthermore, it promotes lipid breakdown in adipocytes, releasing free fatty acids and subsequently inhibiting the insulin signaling pathway, ultimately contributing to insulin resistance [42]. PPAR signaling pathway was involved in many physiological processes of HLP and diabetes-related cardiovascular diseases, including inflammatory response, energy metabolism, lipid metabolism, and so on [43].
This study offers preliminary insights into the potential active ingredients of Ganoderma lucidum that exhibit anti-HLP effects and their underlying mechanisms of action. These findings serve as a foundation for further research on anti-HLP bioactive compounds and facilitate the development of traditional Chinese medicine components for managing HLP. While the network pharmacology approach facilitated the prediction of Ganoderma lucidum’s anti- HLP mechanism in this study, definitive validation necessitates additional experimental investigations and clinical applications.
Conclusion
By integrating network pharmacology and bioinformatics, this study elucidated the material basis of Ganoderma lucidum anti-HLP. It was found that Ganoderma lucidum may promote the activation of blood lipids through core components such as valeric acid, cerevisterol, β-sitosterol, etc. on targets such as TNF, PPARG, PPARA, HIF1A, ESR1, EGFR, CYP3A4, etc. Regulate TNF, PPAR and other signaling pathways to play an anti-HLP role. This study elucidated the molecular mechanism of Ganoderma lucidum action on HLP, and provided a reference for further investigation of the mechanism of anti-HLP.
Consent for Publication
All authors have declared their consent for this publication.
Funding
This work was supported by the Anhui Polytechnic University Introduced Talent Research Start-up Fund (Grant. S022024015); Wuhu Green Food Industry Research Institute Co., Ltd. (Grant. LSSPCYYJY-2024KF-05); Innovative training program for college students (Grant. S202410363241).
Declaration of Competing Interest
No potential conflict of interest was reported by the authors.
CRediT Authorship Contribution Statement
Chuan-chao Wu: Investigation, Data curation, Conceptualization, Formal analysis, Writing-original draft, Funding acquisition, Writing-Review & Editing; Jun-Qiang Dong: Writing-review & editing, Resources; Long Qian: Formal analysis, Software; Xiao-Ting Zheng: Investigation, Methodology; Yu Chen and Qing-Tao Liu: Project administration, Funding acquisition; Zheng-Lian Xue: Writing- review & editing, Project administration.
References
- Subhadip Banerjee, Amrendra Tiwari, Amit Kar, Joydeb Chanda, Sayan Biswas, et al. (2023) Combining LC-MS/MS profiles with network pharmacology to predict molecular mechanisms of the hyperlipidemic activity of Lagenaria siceraria stand. J Ethnopharmacol 300: 115633.
- Peipei Hong, Yang Gao, Qiuyue Wang, Xianliang Qiu, Qiu Chen, et al. (2020) The effectiveness of acupoint catgut embedding in hyperlipidemia with obesity: Protocol for a systematic review and meta-analysis. Medicine 99(22): 20342.
- Livia Pisciotta, Stefano Bertolini, Aldo Pende (2015) Lipoproteins, Stroke and Statins. Curr Vasc Pharmacol 13(2): 202-208.
- Yuchen Liu, Dongsheng Tan, Hong Cui, Jihua Wang (2023) Ganoderic acid C2 exerts the pharmacological effects against cyclophosphamide-induced immunosuppression: a study involving molecular docking and experimental validation. Sci Rep 13(1): 17745.
- Bojana Boh, Marin Berovic, Jingsong Zhang, Lin Zhi Bin (2007) Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol Annu Rev 13: 265-301.
- Bhagwan S Sanodiya, Gulab Singh Thakur, Rakesh K Baghel, G B K S Prasad, P S Bisen (2009) Ganoderma lucidum: A Potent Pharmacological Macrofungus. Curr Pharm Biotechnol 10(8): 717-742.
- Kexue Zhu, Shaoping Nie, Chuan Li, Suli Lin, Mengmeng Xing, et al. (2013) A newly identified polysaccharide from Ganoderma atrum attenuates hyperglycemia and hyperlipidemia.Int J Biol Macromol 57: 142-150.
- L HA (2007) Network pharmacology. Nat Biotechnol. 25(10): 1110-1111.
- Lin Han, Xiu Xiu Wei, Yu Jiao Zheng, Li Li Zhang, Xin Miao Wang, et al. (2020) Potential mechanism prediction of Cold-Damp Plague Formula against COVID-19 via network pharmacology analysis and molecular docking. Chin Med 15: 78.
- Long Lv, Jinghu Du, Daorong Wang, Zeqiang Yan, et al. (2024) A Comprehensive Study to Investigate the Tumor-Suppressive Role of Radix Bupleuri on Gastric Cancer with Network Pharmacology and Molecular Docking.Drug Des Dev Ther 18: 375-394.
- Wenfei Zheng, Manshu Lei, Yao Yao, Jingqiong Zhan, Yiming Zhang, et al. (2024) Mechanisms underlying the therapeutic effects of Semen cuscutae in treating recurrent spontaneous abortion based on network pharmacology and molecular docking. Front Mol Biosci 11: 1282100.
- Xia Shen, Zhenyu Zhao, Hao Wang, Zihu Guo, Benxiang Hu, et al. (2017) Elucidation of the Anti-Inflammatory Mechanisms of Bupleuri and Scutellariae Radix Using System Pharmacological Analyses. Mediators Inflamm 2017: 3709874.
- Xue Xu, Wuxia Zhang, Chao Huang, Yan Li, Hua Yu, et al. (2012) A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci 13(6): 6964-6982.
- Bo Ma, Jinyu Ning, Fengyun Wang, Huiling Zheng, Liang Han, et al. (2023) Molecular Mechanism of the Therapeutic Effect of Peach Blossom against Constipation: An Exploratory Study Based on Network Pharmacology Analysis and Molecular Docking Verification.Evid-Based Complement Alternat Med 2023: 8577485.
- Gil Stelzer, Naomi Rosen, Inbar Plaschkes, Shahar Zimmerman, Michal Twik, et al. (2016) The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr Protoc Bioinformatics 54: 1.30.1-1.30.33.
- Janet Piñero, Núria Queralt Rosinach, Àlex Bravo, Jordi Deu Pons, Anna Bauer Mehren, et al. (2015) DisGeNET: a discovery platform for the dynamical exploration of human diseases and their genes. Database 2015: bav028.
- Joanna S Amberger, Carol A Bocchini, François Schiettecatte, Alan F Scott, Ada Hamosh, et al. (2014) OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an Online catalog of human genes and genetic disorders. Nucleic Acids Res 43: 789-798.
- Changlin Zhang, Yingdi Liao, Lingling Liu, Yifan Sun, Shaoqin Lin, et al. (2020) A Network Pharmacology Approach to Investigate the Active Compounds and Mechanisms of Musk for Ischemic Stroke. Evid-Based Complement Alternat Med 2020: 4063180.
- Nadezhda T Doncheva, John H Morris, Jan Gorodkin, Lars J Jensen (2019) Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J Proteome Res 18(2): 623-632.
- Fei Liao, Muhammad Yousif, Ruya Huang, Yanlong Qiao, Yanchun Hu, et al. (2023) Network pharmacology- and molecular docking-based analyses of the antihypertensive mechanism of Ilex kudingcha. Front Endocrinol 14: 1216086.
- Glynn Dennis, Brad T Sherman, Douglas A Hosack, Jun Yang, Wei Gao, et al. (2003) DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4(5): P3.
- Lin-Zi Li, Hui Ying Wang, Jia Hui Huang, Kun Liu, Xiao Jie Feng, et al. (2022) The Mechanism of Dendrobium officinale as a Treatment for Hyperlipidemia Based on Network Pharmacology and Experimental Validation. Evid-Based Complement Alternat Med 2022: 5821829.
- Shasha He, Xuhua He, Shujuan Pan, Wenwen Jiang (2023) Exploring the Mechanism of Chuanxiong Rhizoma against Thrombosis Based on Network Pharmacology, Molecular Docking and Experimental Verification. Molecules. 28(18): 6702.
- Da Wei Huang, Brad T Sherman, Richard A Lempicki (2008) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4(1): 44-57.
- Ain Nabila Syahira Shamsol Azman, Jun Jie Tan, Muhammad Nazrul Hakim Abdullah, Hasnah Bahari, Vuanghao Lim, et al. (2023) Network Pharmacology and Molecular Docking Analysis of Active Compounds in Tualang Honey against Atherosclerosis. Foods 12(9): 1779.
- Kun Yi H (2013) Combining Machine Learning Systems and Multiple Docking Simulation Packages to Improve Docking Prediction Reliability for Network Pharmacology. Plos One 8: 1-9.
- Hua Chen, Hua Miao, Ya Long Feng, Ying Yong Zhao, Rui Chao Lin, et al. (2014) Metabolomics in dyslipidemia. Adv Clin Chem 66: 101-119.
- Wei Yang, Yan Tian, Mingmao Yang, John Mauck, Juan J Loor, et al. (2024) β-sitosterol alleviates high fatty acid-induced lipid accumulation in calf hepatocytes by regulating cholesterol metabolism. J Steroid Biochem Mol Biol 243: 106543.
- Jing Ge, Jun Jun Miao, Xin Yi Sun, Jiang Yi Yu (2016) Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (PPAR)-alpha/gamma and attenuating endoplasmic reticulum stress in rats.J Ethnopharmacol 189: 238-249.
- Christopher E Lowe, Stephen O Rahilly, Justin J Rochford (2011) Adipogenesis at a glance. J Cell Sci 124: 2681-2686.
- Farmer SR (2006) Transcriptional control of adipocyte formation. Cell Metab 4(4): 263-273.
- Lehrke M, Lazar MA (2005) The many faces of PPARgamma. Cell 123(6): 993-999.
- Siersbaek R (2010) PPARγ in adipocyte differentiation and metabolism-Novel insights from genome-wide studies. Febs Lett 584(15): 3242-3249.
- Lee JE, Ge K (2014) Transcriptional and epigenetic regulation of PPARγ expression during adipogenesis. Cell Biosci 4: 29.
- João Nilton Barreto Andrade, Luciana Alves de Fátima, Raquel Saldanha Campello, José Augusto Cipriano Guedes, Helayne Soares de Freitas, et al. (2018) Estrogen receptor 1 (ESR1) enhances Slc2a4/GLUT4 expression by a SP1 cooperative mechanism. Int J Med Sci 15(12): 1320-1328.
- L Cominacini, A F Pasini, U Garbin, A Davoli, M L Tosetti, et al. (2000) Oxidized Low-Density Lipoprotein (ox-LDL) Binding to ox-LDL Receptor-1 in Endothelial Cells Induces the Activation of NF-κappaB through an Increased Production of Intracellular Reactive Oxygen Species. J Biol Chem 275(17): 12633-12638.
- Sorim Choung, Ji Min Kim, Kyong Hye Joung, Eaum Seok Lee, Hyun Jin Kim, et al. (2019) Epidermal growth factor receptor inhibition attenuates non-alcoholic fatty liver disease in diet-induced obese mice. Plos One 14(2): 0210828.
- Lingxia Zhao, Xuemei Fan, Lin Zuo, Qiang Guo, Xiaole Su, et al. (2018) Estrogen receptor 1 gene polymorphisms are associated with metabolic syndrome in postmenopausal women in China. Bmc Endocr Disord 18(1): 65.
- James R Reed, Jessie J Guidry, Marilyn Eyer, Wayne L Backes (2023) The Influence of Lipid Microdomain Heterogeneity on Protein-Protein Interactions: Proteomic Analysis of Co-Immunoprecipitated Binding Partners of P450 1A2 and P450 3A in Rat Liver Microsomes. Drug Metab Dispos 51(9): 1196-1206.
- Pei Li, Jiaxian Zhang, Jinyun Wu, Juanqiong Ma, Wenyi Huang, et al. (2024) Integrating serum pharmacochemistry and network pharmacology to reveal the mechanism of chickpea in improving insulin resistance. Fitoterapia 172: 105750.
- Jiami Huang, Xu Zhang, Jiayun Wang, Cancan Gu, Yanan Zhang, et al. (2023) Mechanism of Yushenhuoxue prescription in treating endometriosis based on network pharmacology and the effect on the TNF pathway. Heliyon 9(10): 20283.
- Xiaoning Zhang, Jie Shi, Yilong Lu, Rui Ji, Zhiyu Guan, et al. (2024) Mechanism of oxymatrine in the treatment of cryptosporidiosis through TNF/NF-κB signaling pathway based on network pharmacology and experimental validation. Sci Rep 14(1): 14469.
- Gao T (2024) Identification and functional prediction of new triterpenoids from Alismatis Rhizoma using HPLC-HRMS and in-silico analysis. Arab J Chem 17: 1-14.









 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.