Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Bioinspired Iron (III) Complexes: Catalysts for Sustainable Oxygen Atom Transfer Reactions
*Corresponding author: prof. Dr. Nasser Thallaj Pharmaceutical chemistry and drug quality control Department, Faculty of Pharmacy, Al-Rachid privet University, Damascus, Syria. ORCID ID: 0000-0002-6279-768X.
Received: February 14, 2025; Published: February 19, 2025
DOI: 10.34297/AJBSR.2025.27.003516
Abstract
This review focuses on bioinspired semi-hemic iron (III) complexes as innovative catalysts for chemical and photochemical Oxygen Atom Transfer (OAT) reactions. Endosulfatases, particularly HSulf1 and HSulf2, play critical roles in regulating Heparan Sulfate (HS) sulfation, influencing various biological processes and disease mechanisms, including cancer and inflammation. We examine the synthesis and characterization of novel dipyrrin-based iron complexes, which stabilize high-valent iron-oxo intermediates essential for OAT. Utilizing environmentally friendly oxidants such as dioxygen and water aligns with sustainable chemistry principles, promoting efficient catalytic systems.The mechanisms of OAT reactions are elucidated through spectroscopic techniques, revealing insights into the interactions between iron centers and substrates. Challenges related to the instability of high-valent iron species are addressed, highlighting innovative activation strategies that enhance their reactivity and stability. Additionally, the review discusses current inhibitor strategies targeting endosulfatases, particularly sulfamate-containing compounds, which show promise in modulating HS sulfation patterns. By advancing the understanding of iron-mediated catalysis and the development of specific inhibitors, this research underscores the potential for therapeutic applications in diseases characterized by aberrant HS interactions. Future directions aim to refine inhibitor potency and selectivity while exploring targeted drug delivery methods. This exploration not only enhances our comprehension of iron-catalyzed oxidation processes but also paves the way for innovative solutions in glyco-chemistry and biomedicine.
Keywords: Bioinspired, Iron (III) Complexes, Oxygen Atom Transfer (OAT), Catalysis, High-Valent Iron-Oxo Species, Sustainable Chemistry, Dipyrrin
Introduction
The exploration of transition metal complexes for catalysing oxidation reactions has garnered significant attention in recent years, particularly within the field of green chemistry. Among these, iron complexes have emerged as promising candidates due to their abundance, low toxicity, and ability to mimic the reactivity of natural metalloenzymes [1]. Oxidation reactions involving Oxygen Atom Transfer (OAT) and hydrogen atom abstraction are fundamental transformations in organic synthesis, yet they often rely on precious and toxic metals such as palladium, iridium, and rhodium, along with hazardous oxidizing agents [2]. This reliance poses both economic and environmental challenges, highlighting the urgent need for the development of sustainable catalytic systems. Natural enzymatic systems, particularly those involving iron, have evolved to efficiently perform complex oxidation reactions under mild conditions, making them ideal models for synthetic chemists [3]. Enzymes such as cytochrome P450, α-ketoglutarate-dependent hydroxylases, and soluble methane monooxygenases (sMMO) exemplify how nature has optimized metal-centered catalysis to activate molecular oxygen and facilitate OAT processes. These enzymes activate dioxygen to form high-valent metal-oxo species, which are highly reactive and capable of transferring oxygen atoms to a wide range of substrates, thereby demonstrating exceptional selectivity and efficiency [4].
Cytochrome P450 enzymes, for instance, utilize a heme group containing an iron atom coordinated to a thiolate ligand. This unique structure allows them to effectively hydroxylate inert hydrocarbons such as cyclohexane, a feat that remains a challenge for synthetic catalysts. The catalytic cycle of cytochrome P450 involves the formation of a high-valent iron-oxo species, often referred to as Compound I, which is pivotal in the mechanism of oxygen activation and substrate oxidation [5]. The intrinsic reactivity of this species underscores the potential for synthetic models to replicate such catalytic behavior. In recent years, the biomimetic approach to designing synthetic catalysts has gained traction [6]. By replicating the structural and electronic features of natural enzymes, researchers have developed iron complexes that can efficiently catalyze oxidation reactions. One notable strategy involves the use of porphyrin- based ligands, which closely resemble the heme structure found in cytochrome P450. These synthetic metalloporphyrins have been shown to facilitate OAT and hydrogen abstraction reactions, providing valuable insights into the mechanistic pathways of iron-mediated catalysis [7-12].
However, the development of synthetic systems that can compete with the efficiency and selectivity of natural enzymes remains a formidable challenge. The design of ligands that can stabilize high-valent iron-oxo species while maintaining reactivity is crucial. Researchers have explored various ligand frameworks, including biomimetic and bioinspired designs, to enhance the catalytic performance of iron complexes. Biomimetic ligands aim to closely resemble the coordination environment of natural enzymes, while bioinspired ligands focus on capturing the essential features that facilitate OAT and hydroxylation reactions. Recent advancements in synthetic methodologies have led to the creation of robust iron catalysts capable of activating dioxygen in the presence of mild reducing agents. For instance, the integration of electron-rich ligands has been shown to stabilize high-valent iron species, enhancing their reactivity towards substrates. Additionally, the use of environmentally friendly oxidants, such as hydrogen peroxide and molecular oxygen, aligns with the principles of green chemistry, promoting sustainability in chemical processes. Despite these advancements, the quest for effective synthetic iron catalysts continues [13-17]. The instability of high-valent iron-oxo intermediates presents significant challenges for characterization and application. Spectroscopic techniques, including Mössbauer spectroscopy, EPR, and resonance Raman spectroscopy, play a vital role in elucidating the electronic structure and reactivity of these species. Understanding the fundamental mechanisms behind OAT and hydrogen abstraction reactions is essential for the design of next-generation catalysts that can efficiently perform these transformations. In this context, the present study aims to explore the synthesis and characterization of bioinspired iron complexes that can facilitate OAT and hydroxylation reactions [17-22]. By leveraging recent developments in ligand design and synthetic methodologies, we seek to develop iron catalysts that not only mimic the reactivity of natural enzymes but also exhibit enhanced stability and catalytic efficiency. The incorporation of novel ligands, such as dipyrrin-based scaffolds, offers a unique platform for stabilizing high-valent iron species, paving the way for new insights into iron-mediated catalysis [22-28]. The development of bioinspired iron complexes for OAT reactions represents a significant step towards sustainable catalysis. By harnessing the principles of nature and advancing synthetic methodologies, we aim to contribute to the ongoing efforts to create more efficient and environmentally friendly catalytic systems. The findings of this research will not only enhance our understanding of iron-mediated catalysis but also provide a foundation for the design of future catalysts that can address the pressing challenges in chemical synthesis [28-32].
Enzymatic Systems Facilitating Oxidation in Nature
Catalytic enzymes that mediate oxidation reactions can be categorized into two primary groups: heme-containing proteins, such as cytochrome P450, and non-heme proteins, which include soluble methane monooxygenases and α-ketoglutarate-dependent hydroxylases (Table 1).
Cytochrome P-450
Cytochrome P-450 enzymes represent a crucial family of heme (iron porphyrin) metalloenzymes found across diverse life forms, including bacteria, plants, yeasts, insects, and mammals. These heme-containing enzymes function as monooxygenases, capable of activating dioxygen and incorporating an oxygen atom into a wide array of substrates under mild conditions. Cytochrome P-450s facilitate reactions such as aromatic and aliphatic hydroxylation, olefin epoxidation, and sulfoxide formation, utilizing dioxygen as the oxygen source and Nicotinamide Adenine Dinucleotide Phosphate (NADPH) as the reducing agent. The high selectivity exhibited by cytochrome P-450s in these reactions is particularly fascinating to chemists, as they are adept at hydroxylating relatively inert substrates like cyclohexane without compromising the integrity of the delicate enzyme structure [32-38].
The active site of cytochrome P-450 enzymes (Figure 1) features an iron protoporphyrin IX core, coordinated to a thiolate from a cysteine residue in the axial position. The remaining coordination site is typically occupied by a labile molecule, such as a solvent molecule, enabling the system to effectively activate dioxygen at this site [38-41] (Figure 1).
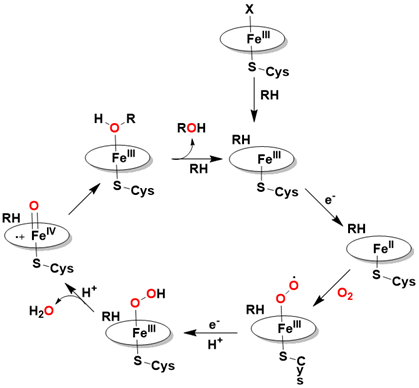
Mechanism of Cytochrome P-450
The key features of the cytochrome P-450 mechanism are depicted in Scheme I-1. The catalytic process begins with substrate binding to the enzyme, resulting in the displacement of a labile ligand and potentially inducing a change in the spin state of the iron center. Molecular oxygen then coordinates to the reduced Fe (II) form of cytochrome P-450, which is generated by the initial reduction of the iron (III) state through the action of the reducing agent NADPH. This is followed by a second one-electron reduction and protonation, leading to the formation of the Fe (III)-hydroperoxo species [42-47].
Subsequently, protonation and heterolytic cleavage of the O-O bond yield a high-valent iron(IV)-oxo porphyrin cation radical, known as Compound I. This intermediate has been identified and characterized in various heme enzymes through multiple spectroscopic techniques, including Mössbauer spectroscopy (δ = 0.05- 0.14 mm/s; ΔEQ = 0.90-1.33 mm/s, indicative of a low-spin (S = 1) FeIV species), EPR spectroscopy (g ≈ 2, associated with radical species), and Raman spectroscopy (750-790 cm⁻¹, attributed to Fe-O vibrational modes) [46-50].
Ultimately, the transfer of an oxygen atom from this iron-oxo species to the bound substrate results in the formation of the oxygenated product. Computational studies suggest that the radical character may be partially localized on the thiolate ligand or other protein residues. Nonetheless, the iron (IV)-oxo porphyrin radical intermediate is widely regarded as the primary reactive species responsible for the activity of cytochrome P-450s, supported by various spectroscopic investigations Scheme 1.
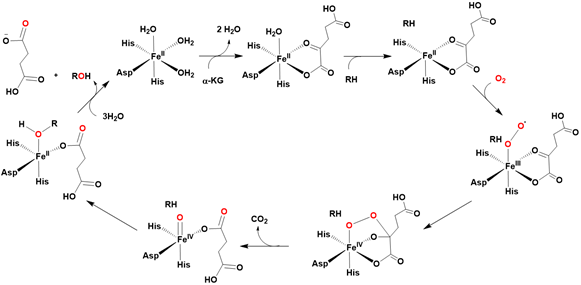
α - Ketoglutarate Dependent Hydroxylases
Iron (II) and α-ketoglutarate-dependent hydroxylases are mononuclear non-heme metalloenzymes that catalyze a remarkably diverse array of hydroxylation reactions, utilizing α-ketoglutarate as a co-substrate. These enzymes feature an active site in which a single iron (II) center is coordinated by three water molecules and three amino acid side-chain ligands (Figure 2).
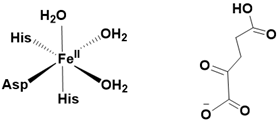
Figure 2: Active site of α - ketoglutarate dependent hydroxylases (left) and α - ketoglutarate (right).
The activation of dioxygen, via the oxidative decarboxylation of α-ketoglutarate, results in the formation of a highly reactive iron (IV)-oxo (ferryl) species. This activated intermediate is responsible for hydroxylating a variety of substrates, including methylated nucleotides and lipids [51-55].
The currently accepted mechanism for this catalytic reaction is based on spectroscopic investigations of enzymes like Clavaminate Synthase 2 and Taurine dioxygenase. Notably, in 2003, Bollinger and Krebs provided definitive spectroscopic evidence for a nonheme high-spin (S = 2) iron (IV)-oxo species in Taurine dioxygenase, using Mössbauer spectroscopy [55-59].
This high oxidation state of the iron center was characterized by an isomer shift (δ) of 0.31 mm/s and a quadrupole splitting (ΔEQ) of 0.88 mm/s. Further resonance Raman studies identified an Fe-O vibration at 821 cm⁻¹, which shifted to 787 cm⁻¹ upon labeling with ¹⁸O Figure 3.
Soluble methane monooxygenases
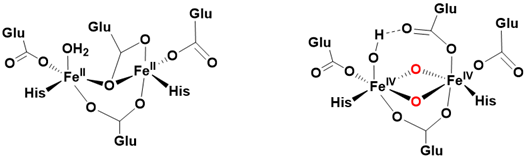
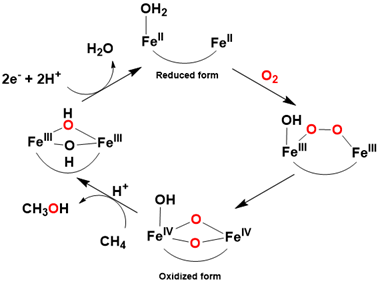
Soluble methane monooxygenases (sMMOs), found in certain bacteria, exhibit similarities to cytochrome P-450s and α-ketoglutarate-dependent hydroxylases in their capacity to hydroxylate unactivated C-H bonds of methane (BDE C-H = 105 kcal/ mol), utilizing dioxygen as the oxygen source and NADP as the reducing agent. sMMOs possess dinuclear iron units within their active site, each situated in a non-heme environment (Figure 4).
In contrast to mononuclear metalloenzymes, sMMO’s active form features a bis-μ-oxo diiron (IV) species, characterized by a diamond core structure, which has been isolated and spectroscopically characterized during catalysis. The catalytic mechanism involves the activation of dioxygen by both iron centers, leading to the formation of a peroxo bridge between two iron (III) ions. Subsequently, homolytic cleavage of the O-O bond generates the active bis-μ-oxo diiron (IV) species, which is then capable of reacting with methane Figure 5.
In brief, biological enzymes can oxygenate hydrocarbons under ambient conditions by activating dioxygen to generate high-valent iron-oxo species, utilizing reducing agents (NADP) or cofactors such as α-ketoglutarate. While these natural metalloenzymes exhibit remarkable efficiency and selectivity in hydrocarbon oxidation, their isolation from biological sources, such as plants, is exceedingly challenging. Indeed, the genes encoding cytochrome P-450s, for instance, were only recently isolated. Consequently, the development of robust model systems that mimic these metalloenzymes is crucial to overcome the inherent difficulties associated with working directly with the enzymes. Furthermore, investigations of synthetic model systems provide valuable insights into the fundamental nature of enzymatic processes [60-67].
Synthetic iron catalysts for oxidation reaction
Once the metal center is selected, chemists must consider two key parameters that influence catalyst reactivity: the ligand, which modulates the electronic and steric properties of the metal center, and thereby the activation strategies. These two factors are discussed below [67-73].
Ligand design
In recent years, the development of efficient synthetic systems that mimic catalytic metalloenzymes for oxidation reactions has garnered significant attention. The catalytic prowess of metalloenzymes is largely attributed to the protein environment, and particularly the structural ligands that coordinate the metal center. Synthetic ligands can be categorized into two groups: biomimetic ligands and bioinspired ligands, based on their design strategy [73- 79].
Biomimetic approach
The development of efficient synthetic systems that mimic catalytic metalloenzymes for oxidation reactions has garnered significant attention in recent years. The catalytic efficiency of metalloenzymes is largely attributed to the protein environment, particularly the structural ligands that coordinate the metal center. Synthetic ligands can be classified into two categories: biomimetic and bioinspired, based on their design strategy. A biomimetic ligand is a structural analog that exhibits spectroscopic features similar to those of the enzyme’s cofactor. For example, porphyrin, a wellknown biomimetic ligand, was developed by replicating the ligand core of protoporphyrin IX, found at the active site of Cytochrome P-450s, to mimic a similar electronic density with four nitrogen coordination sites (Figure 1-6).
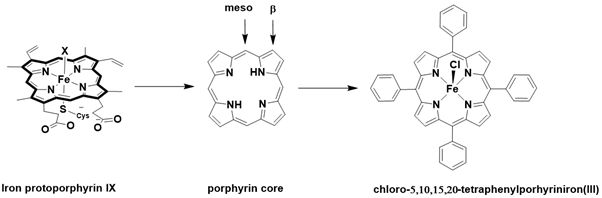
Figure 6: Porphyrin core used for synthetic porphyrin ligand and formula of metalloporphyrin of the first generation used in model system of Cytochrome P450s.
The use of metalloporphyrins as catalysts has received increasing attention over the past four decades, since Groves and co-workers reported the first synthetic metalloporphyrin, chloro-5,10,15,20-tetraphenylporphyriniron (III) (FeIIITPPCl), in 1979, which catalyzed alkene epoxidation and alkane hydroxylation using iodosylbenzene as an oxygen donor. Subsequent generations of metalloporphyrins, with substitutions at the meso and β positions, have demonstrated enhanced efficiency in oxidation reactions (Figure 6). The influence of these distal substituents on reactivity will be discussed in the following paragraph [78-84].
One of the notable characteristics of cytochrome P-450 enzymes is the presence of a thiolate ligand, which significantly influences the reactivity of the heme iron complex. To investigate this effect, synthetic models incorporating a thiolate (RS-) moiety have been developed. To prevent the replacement of the thiolate ligand by other ligands in solution, researchers have utilized a “S-bridge” or “S-tail” to attach the thiolate to the tetraphenylporphyrin framework.
The first report of an iron porphyrin coordinated by an alkylthiolate anion for catalytic oxidation in 1990. His findings indicated that the thiolate ligand enhances the oxidation rate of substrates such as 2,4,6-tri-tert-butylphenol and 1,1-diphenyl-2-picrylhydrazine by approximately 100-fold compared to FeIII tetraphenylporphyrin chloride (FeIIITPPCl), employing alkyl hydroperoxides as oxidants. The oxidation mechanism involves several stages: the formation of an alkyl peroxide iron porphyrin, cleavage of the O-O bond, leading to an active iron-oxo species, and the subsequent reaction between the iron-oxo complex and the substrate [85-86].
This acceleration in the oxidation rate is likely due to the strong electron-donating properties of the alkyl thiolate, which facilitate O-O bond cleavage and stabilize the high-valent iron-oxo species. The electron-donating effect of the thiolate ligand is further evidenced by a significant negative shift of approximately 200 mV in the FeIII/FeII redox potential, as observed through cyclic voltammetry, compared to the iron porphyrin lacking a thiolate ligand (FeIIITPPCl) Figure 7.
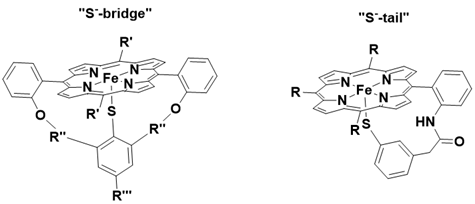
Figure 7: Synthetic metalloporphyrins containning a « S-- bridge » ligand (left) and « S-- tail » ligand (right).
Despite the promising findings from Hirobe’s group, the advancement of synthetic heme metalloporphyrins containing thiolate ligands faced significant challenges due to the susceptibility of thiolate ligands to oxidation, resulting in the formation of disulfides. The presence of an electron-donating thiolate ligand compromises the stability of these synthetic complexes under strongly oxidizing conditions. Consequently, these models did not receive further investigation [87].
In contrast, the incorporation of electron-withdrawing groups (EWGs) at the meso or β positions has been shown to enhance the reactivity of cyclohexene oxidation, as illustrated in Table 2 and Figure 8. EWGs increase the electrophilic character of the Fe=O moiety, thereby improving the reactivity of the catalysts when interacting with electron-rich nucleophiles, such as alkenes. Furthermore, the introduction of EWGs contributes to better stability of the catalysts against oxidative degradation [88].
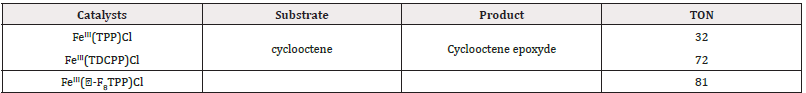
Table 2: Cyclohexene epoxidation by PhIO catalyzed by iron porphyrin complex. Cyclohexene/PhIO/catalyst = 100/100/1, solvent : DCM. TON based on PhIO in 2h.
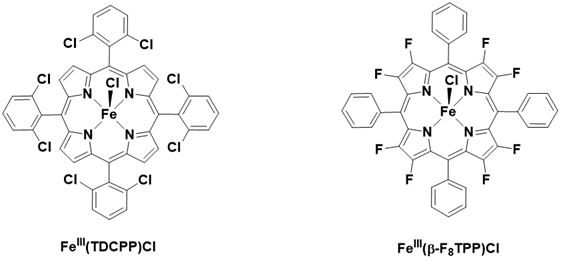
Figure 8: Structure of iron (III) porphyrins with electron withdrawing group in meso and α position.
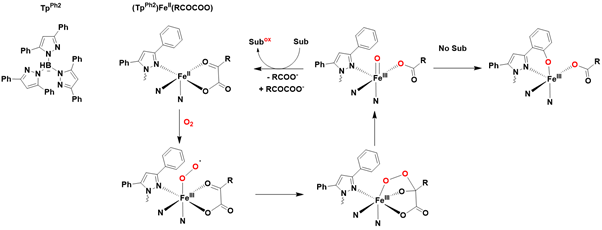
Over the past two decades, several biomimetic non-heme models of metalloenzymes have been developed to facilitate the generation of iron-oxo species capable of catalyzing oxidation reactions. In 2003, a synthetic model for α-ketoglutarate-dependent hydroxylases was introduced, utilizing a tridentate ligand known as TpPh2 (hydrotris(3,5-diphenylpyrazol-1-yl)borate) to mimic the coordination of three amino acid ligands [89].
An α-keto acid was employed as a sacrificial electron donor, supplying the two electrons necessary for dioxygen activation. This non-heme model demonstrated the ability to react with dioxygen, leading to oxidative decarboxylation of the α-keto acid, and exhibited versatile reactivity, including sulfide oxidation, alkene cis-hydroxylation, and alkane hydroxylation. Notably, in the absence of any organic substrate, intramolecular hydroxylation of the phenyl group in the TpPh2 ligand was also observed, likely due to the proximity of the phenyl group to the iron center [90]. However, attempts to trap or characterize intermediates from these reactions were unsuccessful Figure 9.
The biomimetic approach involves the replication of structural features from biological systems to develop effective oxidation catalysts for the oxygenation of alkenes and alkanes. Although these catalysts demonstrate moderate catalytic reactivity, primarily due to their limited lifespan under oxidative conditions, they provide valuable insights into the functioning of natural catalytic metalloenzymes. Partial reproduction of enzyme structures, while lacking the intricate protein environment, alters the properties of the active site. Nonetheless, the study of biomimetic systems enhances our understanding of the key factors that contribute to the exceptional catalytic reactivity observed in nature, ultimately guiding the advancement of bioinspired oxidation catalysts [91-97].
Bioinspired Approach
In contrast to the biomimetic approach, the bioinspired strategy focuses on identifying and harnessing the fundamental principles that govern biological systems. A critical component in the mechanisms of iron enzymes is the high-valent iron-oxo intermediate, which is stabilized by complex protein structures. To mimic this stabilization, chemists employ various coordinating functions to support highly oxidized species, predominantly utilizing electron- rich ligands with nitrogen or oxygen binding sites [97-103].
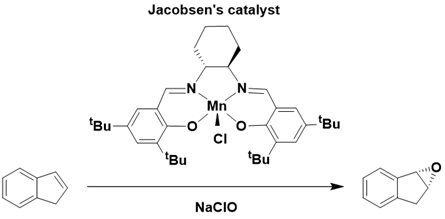
A well-known example of a bioinspired system is the tetradentate Salen ligand, characterized by two phenolic and two iminic binding groups. This configuration allows for two coordination sites on the metal ion, facilitating the binding of external ligands and the activation of terminal oxidants. These dianionic ligands, similar to porphyrins, effectively stabilize high-valent metal centers.
Their structural and electronic properties can be readily tailored through substituent functionalization, leading to enhanced reactivity and durability in Salen-based catalysts with each new generation. Due to their planar and tetradentate nature, a variety of metal centers, including ruthenium, iron, and manganese, have been employed to investigate Salen-based catalysts. A notable instance is Jacobsen’s catalyst, a manganese complex with a chiral Salen ligand, which is capable of catalyzing the asymmetric epoxidation of alkenes. This catalyst plays a significant role in the industrial synthesis of Indinavir, an HIV protease inhibitor, underscoring the considerable potential of oxidation reactions facilitated by bioinspired systems Figure 10.
Building upon the Salen ligand and the macrocyclic tetraamine ligand initially described by Margerum, the TetraAmido Macrocyclic Ligands (TAMLs) represent a prominent class of ligands that have been systematically enhanced over four decades by researchers, notably the groups led by Collins and Gupta. TAMLs are characterized as tetraanionic ligands that create a strong electron-donor ligand field, effectively stabilizing highly oxidized metal centers [104-110].
The structural design of TAMLs incorporates geometric cyclic rigidity, which significantly contributes to their robustness by inhibiting intramolecular isomerization processes that could otherwise lead to degradation. To date, seven generations of TAMLs have been synthesized, with the fifth generation demonstrating the capability to generate an active iron(V)-oxo species, denoted as [(bTAML)FeV=O]1-, at ambient temperature. This iron(V)-oxo species exhibits notable reactivity in the activation of inert C-H bonds in cyclohexane, achieving high selectivity while maintaining exceptional operational stability Figure 11.
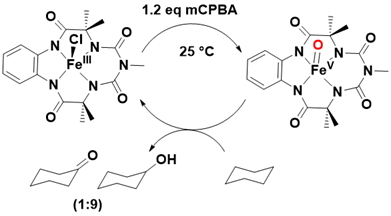
Figure 11: Operationally stable, highly active iron(V)-oxo supported by b-TAML and their reactivity toward cyclohexane oxidation.
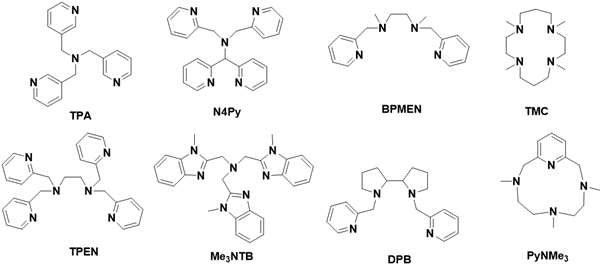
The use of negatively charged ligands, as discussed previously, is not the sole method for generating highly oxidized metal-oxo species. Synthetic neutral polynitrogen ligands, inspired by naturally occurring neutral ligands such as histidine, have also been investigated for this purpose. Since the late 1980s, numerous synthetic systems utilizing polynitrogen ligands have been developed to create active metal-oxo species and catalyze oxidation reactions [111-117].
These polydentate ligands can incorporate π-acceptor nitrogen binding groups such as pyridine, benzimidazole, and imidazole, alongside σ-donor tertiary amines. Their flexible geometries allow them to adapt to the preferred coordination geometry of the metal center. While the absence of a negative charge may reduce the stability of high-valent metal-oxo species, it may also facilitate the formation of more reactive metal-oxo species due to a lower electron density surrounding the metal [117-124].
For instance, in 2011, research demonstrated a nonheme iron (IV)-oxo species supported by the Me3NTB ligand, capable of activating C-H bonds in alkanes and transferring an oxygen atom to thianisole. This study highlighted the first synthetic nonheme iron-oxo species exhibiting greater reactivity than the iron (IV)-oxo porphyrin π-cation radical (Por•) FeIV=O species. The ease of synthesizing and modifying polynitrogen ligands has further enhanced the reactivity of iron-oxo species. Notably, in 2015, an FeV=O species based on the polynitrogen macrocyclic ligand PyNMe3 was reported, demonstrating the highest reactivity in the hydroxylation of cyclohexane among all previously documented iron-oxo species Figure 12.
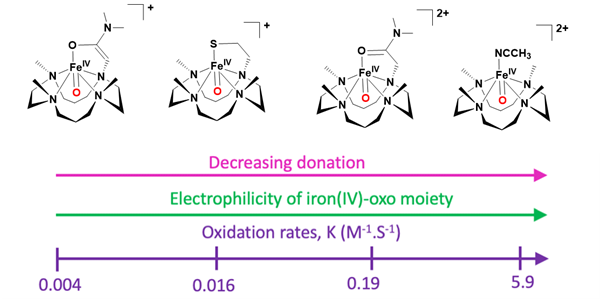
It was demonstrated that altering the nature of the axial ligand in a series of TMC-based complexes allows for the tuning of the electrophilicity and, consequently, the reactivity of iron-oxo species in oxygen atom transfer (OAT) reactions. The OAT process involves a two-electron transfer from the substrate to the iron center, making the electrophilic character of the iron-oxo moiety crucial for this reaction. This electrophilicity can be quantitatively assessed through the reduction potential of the Fen=O/Fen-1=O redox couple. A more electrophilic iron-oxo moiety correlates with increased activity in OAT, as illustrated in the accompanying Figure13.
Activation strategies and mechanistic insights
Using chemical oxygen donor agents
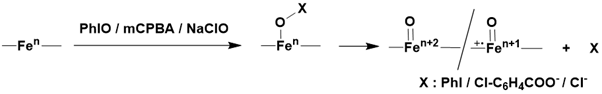
Over the past decade, a variety of synthetic high-valent iron-oxo complexes have been prepared using diverse ligands and methods. The general synthesis involves the oxidation of an iron complex precursor using oxygen atom donor oxidants such as iodosylbenzene, sodium hypochlorite, peracids, and hydroperoxides. Among these oxidants, two-electron oxidants like iodosylbenzene, sodium hypochlorite, and m-chloroperbenzoic acid directly oxidize iron (II) precursors to iron (IV)-oxo species, and iron (III) precursors to either iron (V)-oxo or iron (IV)-oxo ligand cation radical species. The overall mechanism typically occurs through two consecutive steps: first, the formation of an adduct, followed by oxygen atom transfer initiated by heterolytic cleavage of the O-X bond Figure 14.
Numerous studies have employed hydrogen peroxide as an oxidant to gain insights into the mechanisms of O-O bond cleavage in biological systems. In the presence of excess hydrogen peroxide in acetonitrile, an iron(II) precursor is thought to be oxidized by half an equivalent of hydrogen peroxide to form an iron(III) complex [122-126]. This complex then rapidly reacts with another equivalent of hydrogen peroxide to generate a hydroperoxo iron(III) species. The subsequent heterolytic cleavage of the O-O bond leads to the formation of an iron(V)-oxo species. Thus, the generation of an iron(V)-oxo species from an iron(III) precursor requires only one equivalent of hydrogen peroxide.H2O2 Figure 15.
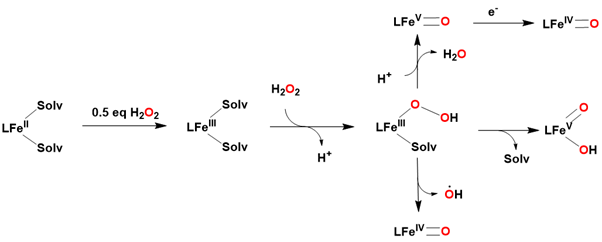
Figure 15: Proposed mechanism for generation of an iron(V)-oxo species from iron (II) precursor using H2O2.
In 2011, reported the complete characterization of the [(FeIV=O) TMC] ²⁺ species, which is generated through the reaction of [FeII(TMC)] ²⁺ with excess hydrogen peroxide in the presence of acid. The rate of formation of [(FeIV=O) TMC ] ²⁺ was found to be dependent on proton concentration. This suggests that the formation process involves proton-assisted heterolytic cleavage of the O-O bond in the FeIIIOOH intermediate, leading to the generation of a short-lived [(FeV=O) TMC] ³⁺ species, which is subsequently reduced by one electron [127].
Alternatively, iron (IV)-oxo species could also be formed through the decomposition of FeIIIOOH via homolytic O-O bond cleavage; however, Density Functional Theory (DFT) calculations indicate that this pathway is not energetically favorable [128].
Additionally, it was reported that the stoichiometric reaction of [FeII(TMC)] ²⁺ and [FeII(TMC-py)] ²⁺ with hydrogen peroxide in the presence of a base yields the corresponding FeIV=O species with a conversion of 90%. This high yield is likely achieved from just one equivalent of hydrogen peroxide, suggesting the formation of an iron (II) peroxo precursor, followed by heterolytic O-O bond cleavage. The formation of the iron (II) peroxo species may be questioned, as the iron (II) precursor can also be oxidized by a single electron transfer from hydrogen peroxide to yield an iron (III) product Figure 16.
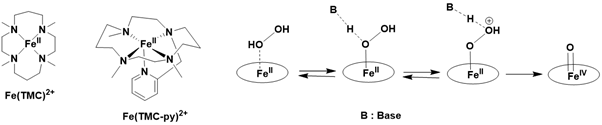
Figure 16: Proposed mechanism for the base-catalyzed formation of iron (IV)-oxo from iron (II) precursor and H2O2.
Using dioxygen as oxygen atom source
Chemical oxidants in controlled quantities enable the generation of active iron species, facilitating the characterization of these short-lived intermediates and the study of mechanistic activation in oxidation reactions. However, the use of environmentally friendly oxygen sources is highly desirable. Dioxygen is recognized as an abundant and sustainable oxygen source, serving as a terminal oxidant in numerous natural oxidation processes catalyzed by both heme and non-heme metalloenzymes [128].
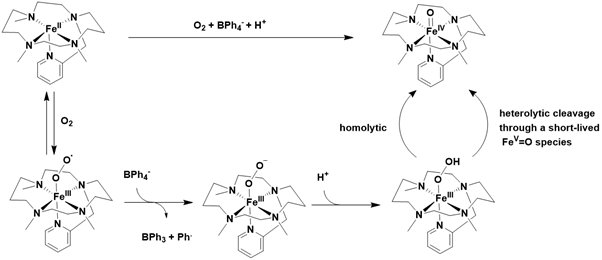
Inspired by cytochrome P-450s, synthetic iron precursors can activate dioxygen to produce active iron-oxo species with the assistance of a proton source and a reducing agent, such as NADPH, BNAH, cobaltocene, or BH₄⁻. For instance, Banse and colleagues demonstrated this promising strategy using the well-known [FeII(TMC-py)]²⁺ precursor, with BPh₄⁻ as the electron source and HClO₄ as the proton source. In the presence of protons and BPh₄⁻, dioxygen is reductively activated at the iron (II) center, resulting in the formation of an iron (III) superoxo species, which subsequently converts into an FeIIIOOH intermediate. This intermediate ultimately decomposes into the iron (IV)-oxo species through O-O bond cleavage Figure 17.
The O-O cleavage can occur via either a homolytic or heterolytic process, followed by rapid decomposition to yield the iron(IV)-oxo species, as previously observed with hydrogen peroxide in acidic conditions. The [FeIV=O(TMC-py)]²⁺ species produced in this study was thoroughly characterized using various spectroscopic techniques, notably X-ray diffraction on single crystals [129] (Figure 17).
The concept of electroactivation of dioxygen was first proposed in earlier studies. In 2013, Dey’s group investigated the activation of dioxygen by iron (III) porphyrin using electrolysis in conjunction with resonance Raman spectroscopy. By employing isotopic labeling with 18O18O, these combined techniques enabled the direct identification of the iron (III) peroxo and iron (IV) oxo intermediates involved in the reduction of the FeII-O₂ adduct at -0.5 V versus Ag/AgCl [130]. However, their findings warrant further scrutiny. In their study, the Fe-O bond of the FeIV=O species was characterized by a vibrational frequency at 780 cm⁻¹. This observation raises questions, as the Fe-O vibrations of all previously reported heme and non-heme iron-oxo species have been experimentally determined to fall within the range of 800-850 cm⁻¹, a point that will be elaborated upon in the following discussion Figure 18.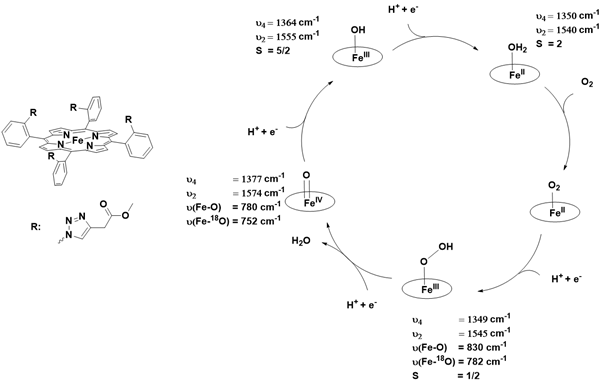
Figure 18: Proposed mechanism for electro-reductive dioxygen activation and the Raman bands of each detected species.
In 2015, an electro-reductive activation method was reported using a non-heme [FeII(TPEN)]²⁺ precursor. The corresponding intermediates, namely [FeIII(OO)(TPEN)]⁺, [FeIII(OOH)(TPEN)]²⁺, and [FeIV(O)(TPEN)] ²⁺, were chemically synthesized in high yields and characterized through electrochemical techniques. A mechanistic investigation of the reaction between [FeII(TPEN)]²⁺ and O₂ was performed using cyclic voltammetry. The analysis and simulation of the resulting data indicated that the iron (III) peroxo species [FeIII(OO)(TPEN)]⁺ is generated by the one-electron reduction of a rarely observed FeII-O₂ adduct at a potential of -0.62 V versus SCE, while dioxygen is reduced to superoxide at a significantly lower potential of -0.87 V. Notably, in contrast to previously studied systems, O-O bond cleavage from the iron(III) peroxo species [FeIII(OO) (TPEN)]⁺ was not detected within the time frame and conditions of the electrochemical analysis Figure 19.
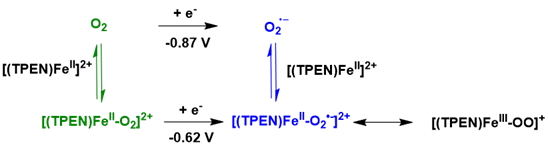
Figure 19: Proposed reductive activation mechanism of O2 at [FeII(TPEN)]2+ based on modelisation of experimental CVs.
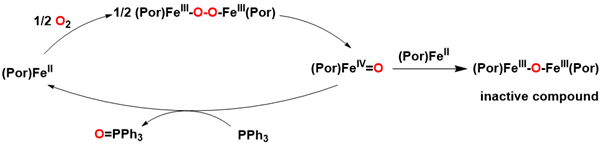
Dioxygen activation can also occur through an autooxidation reaction involving a synthetic catalyst. This approach is particularly appealing as it does not necessitate the presence of an electron donor. The mechanism was elucidated in the 1980s, demonstrating that the reaction proceeds via a μ-peroxo diiron (III) species, which subsequently undergoes homolytic O-O bond cleavage to generate an iron (IV)-oxo intermediate. The μ-peroxo diiron (III) porphyrin was successfully captured and shown to gradually convert to the iron (IV)-oxo species at low temperatures (-80 to -30 °C) using ^1H NMR and UV-visible spectroscopy. In the presence of 50 equivalents of triphenylphosphine (PPh₃), FeIITPP is capable of transferring an oxygen atom to PPh₃, resulting in the formation of O=PPh₃, achieving 27 successful turnover numbers (TON) before inactivation occurs. The deactivation is attributed to the formation of an inactive μ-oxo diiron (III) porphyrin, which was identified in solution at the conclusion of the catalytic reaction Figure 20.
Interestingly, the inactive μ-oxo diiron (III) porphyrin can be reactivated through homolytic photocleavage of the Fe-O bonds using visible light, resulting in the formation of an iron (II) porphyrin and a reactive iron (IV)-oxo intermediate capable of oxygenating various substrates. Research has demonstrated that the μ-oxo diiron (III) porphyrin can serve as an effective catalyst for the photoinduced oxidation of sulfides, olefins, and alkanes in aprotic solvents such as benzene and toluene. Notably, a μ-oxo diiron (III) Pacman porphyrin exhibited significantly greater reactivity compared to its unbridged counterpart, likely due to the facilitated interaction between dioxygen and the two iron centers, which promotes the formation of the μ-peroxo bond through the bridged diporphyrin ligand. However, the precise mechanism underlying the formation of the μ-oxo diiron (III) Pacman porphyrin from dioxygen and diiron (II) Pacman porphyrin remains to be elucidated Figure 21.
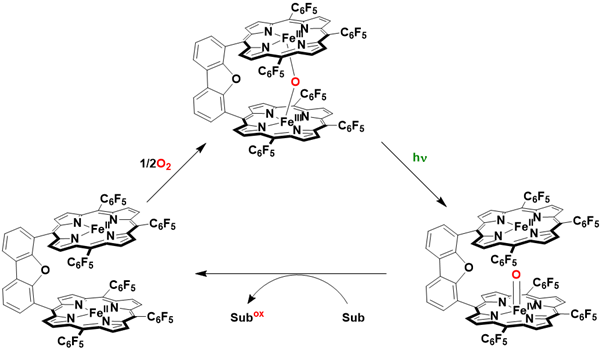
Figure 21: Photocycle of the catalytic oxidation of substrates by μ-oxo diiron(III) Pacman porphyrin using dioxygen without need for an external coreductant.
The non-heme synthetic complex [FeII(TMC)(OTf)₂] is capable of activating dioxygen, likely through the formation of a μ-peroxo diiron (III) intermediate, although its reactivity is moderate. For instance, it converts triphenylphosphine (PPh₃) into O=PPh₃ with a turnover number (TON) of 8, thioanisole into methyl phenyl sulfoxide with a TON of 7, and benzyl alcohol into benzaldehyde with a TON of 6. The potential formation of a μ-oxo diiron (III) complex may represent a deactivation pathway that contributes to this limited reactivity, despite not being directly observed. Additionally, research indicates that the high electronic density of these iron (II) complexes, which exhibit relatively low FeIII/FeII redox potentials (< -0.1 V vs Fc⁺/Fc), is a significant factor in the activation of dioxygen to generate iron (IV)-oxo species. Consequently, other non-heme iron (II) complexes such as [FeII(TPA)]²⁺, [FeII(N4Py)]²⁺, and [FeII(BPMEN)]²⁺ do not react with O₂ to produce iron (IV)-oxo species.
The μ-oxo diiron (IV) 1-TAML complex, first reported by Collins and colleagues in 2004, also demonstrates the ability to oxygenate substrates. The formation of the μ-oxo diiron (IV) 1-TAML complex from the iron (III) 1-TAML complex and dioxygen likely proceeds through μ-peroxo diiron (IV) and iron(V)-oxo intermediates, similar to the mechanism described for the μ-oxo diiron (III) complex. This remarkable reactivity of the iron (III) complex toward dioxygen is likely attributed to the four strongly electron-donating amidato nitrogen ligands, which significantly stabilize the oxidized metal center. The structure of the μ-oxo bis iron (IV) complex has been confirmed through X-ray crystallography. Its reactivity in oxidation reactions is believed to result from the decomposition of the μ-oxo diiron (IV) 1-TAML complex into iron (III) and iron(V)-oxo species in the presence of substrates, with the latter being identified and characterized as the active species. Thus, the μ-oxo diiron (IV) 1-TAML complex can catalytically oxidize a variety of substrates using dioxygen as the source of oxygen atoms Figure 22.
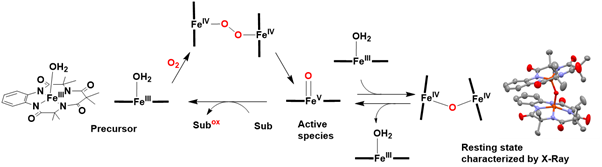
Figure 22: Proposed mechanism for the oxygenation of substrates by dioxygen catalyzed by iron (III) TAML complex in the presence of O2.
Other photocatlytic strategies using a chromophore and an electron donor to photoactivate O2 at an iron center.
Using water as oxygen atom source
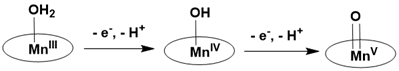
Like dioxygen, water serves as a sustainable source of oxygen atoms, playing a crucial role in the generation of manganese(V)-oxo species during the natural oxidation process of Photosystem II. This enzyme is responsible for water splitting, oxygen evolution, and plastoquinone reduction. Numerous studies on the oxygen-evolving complex within Photosystem II have demonstrated that the Mn- V=O species can be generated through the two-electron oxidation of a water-bound molecule via a proton-coupled electron transfer (PCET) mechanism. In Photosystem II, visible light activates a chromophore, which subsequently oxidizes the manganese-aqua complex to manganese-oxo species through a series of proton-coupled electron transfer reactions Figure 23.
Various synthetic metal systems, including iron-based complexes, have been developed to generate highly oxidized metal-oxo species using water as an oxygen source. In 2009, reported the first synthetic iron-oxo species, [FeIV=O(N4Py)]²⁺, which was generated from [FeII(H₂O)(N4Py)]²⁺ using water as the oxygen source in the presence of cerium(IV) as a strong one-electron oxidant. This species was shown to catalytically oxygenate organic substrates such as thioanisole, benzyl alcohol, cyclohexene, and ethylbenzene. Following this success, developed a photocatalytic system utilizing the same [FeII(H₂O)(N4Py)]²⁺ catalyst, [Ru(bpy)₃]²⁺ as a chromophore, and [CoIII(NH₃)₅]Cl as a sacrificial electron acceptor for similar purposes. This work demonstrated that the active iron-oxo species [FeIV=O(N4Py)]²⁺ can also be produced using a weaker oxidant, [CoIII(NH₃)₅]Cl, through a light-driven reaction with water as the oxygen source Figure 24.
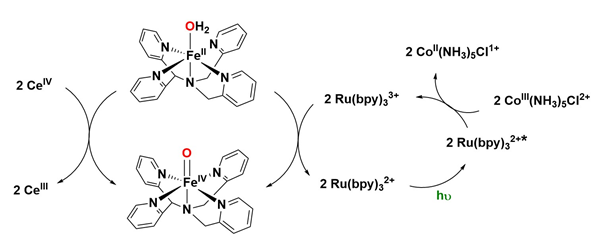
Figure 24: Formation of [FeIV=O(N4Py)]2+ by water chemical activation using Cerium(IV) (left) as an oxidant or by water photoactivation using a chromophore and a sacrificial electron donor (right).
In a similar manner, the iron(V)-oxo species [FeV=O(TAML)]¹⁻ was generated through the oxidation of the corresponding iron (III)-aqua complex [FeIII(OH₂)(TAML)]¹⁻ using cerium(IV) as a strong oxidant, or by light-driven activation with [Ru(bpy)₃]²⁺ serving as a chromophore and sodium dithionite (Na₂S₂O₄) as a sacrificial electron acceptor. Another strategy involves the electroactivation of the metal-bound water molecule. Stahl and colleagues reported the sequential formation of [FeIV(OH)(1-TAML)]¹⁻ and [FeV=O(1-TAML)]¹⁻ species by electrochemically oxidizing [FeIII(OH₂)(1-TAML)]¹⁻ at increasing potentials of 0.34 V and 0.79 V versus Fc⁺/Fc, respectively, demonstrating their catalytic application in the electrochemical oxidation of organic molecules such as alkylbenzenes and benzyl alcohol.
Similarly, a series of high-valent iron (IV)-oxo species, utilizing various non-heme polynitrogen ligands, were quantitatively generated through bulk electrolysis in acetonitrile with water concentrations ranging from 0.1 to 1 M as the oxygen source. The redox behavior of these iron (II) precursors was investigated by cyclic voltammetry in dry acetonitrile, revealing only a FeIII(CH₃CN)/ FeII(CH₃CN) redox couple. In the presence of water, the coordinated acetonitrile was replaced by water, as evidenced by a new reduction wave at a potential more negative than that of the FeIII(CH₃CN)/ FeII(CH₃CN) couple. This feature is attributed to the FeIII/FeII couple of the FeII(OH₂) (L) complex. The iron (II)-aqua precursor undergoes oxidation via two one-electron processes, coupled with a series of deprotonations at different potentials. For instance, [FeII(H₂O)(N4Py)]²⁺ is oxidized during the first one-electron oxidation process at 0.6 V versus Fc⁺/Fc to form [FeIII(OH)(N4Py)]²⁺, and subsequently oxidized to [FeIV=O(N4Py)]²⁺ at 0.9 V versus Fc⁺/ Fc. The potential of the redox couple FeIV=O/FeIII-OH may indicate their oxidative activity toward organic substrates. Indeed, studies by Comba, Costas, and Que demonstrated that higher potential values for this redox couple correlate with increased reactivity of the FeIV=O species in the oxygenation of substrates such as thioanisole, benzyl alcohol, and 1,3-cyclohexadiene.
Quest for the Active Iron Species
High-valent iron-oxo species have been the subject of extensive research over the past few decades to elucidate the chemistry of active sites in enzymes and to enhance the efficiency of synthetic environmentally friendly catalysts for oxidation reactions, including alkene epoxidation and alkane hydroxylation. These iron-oxo species can be characterized using a variety of spectroscopic techniques, such as absorption spectroscopy, Mössbauer spectroscopy, electron paramagnetic resonance (EPR), Raman spectroscopy, nuclear magnetic resonance (NMR), and mass spectrometry (MS).
The oxidation state of iron and the ligand field are primarily assessed through Mössbauer and EPR spectroscopy. Raman resonance spectroscopy is commonly employed to investigate the vibrational energy of the Fe=O bond. While mass spectrometry can provide insights into the structure of iron-oxo species, X-ray diffraction offers precise structural determinations. However, the inherent instability of these highly active high-valent iron-oxo species often hinders their crystallization, even at low temperatures. Additionally, data obtained from extended X-ray absorption fine structure (EXAFS) studies are valuable for characterizing the oxidation state of the iron center and the ligand environment, including the Fe-O bond distances Figure 25.
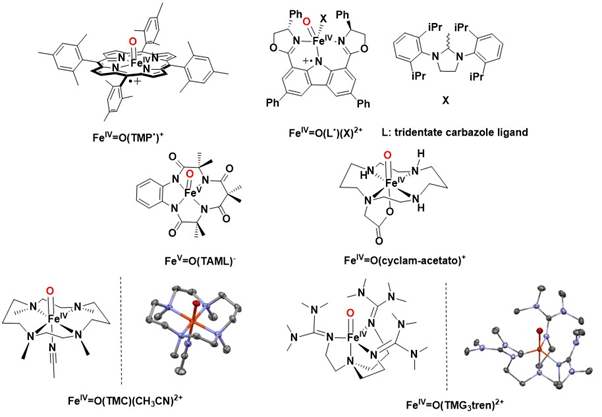
Figure 25: Structure of selected iron-oxo species with their ORTEP plot of their X-ray diffraction analysis when performed.
The iron(IV)-oxo porphyrin cation radical species [FeIV=O(TMP+•)] ⁺ (TMP = 5,10,15,20-tetramesitylporphyrin), which serves as a model for the Compound I intermediate in cytochrome P-450, was first characterized in 1981. This species was synthesized by oxidizing the precursor [FeIII(TMP)Cl] with mCPBA in a DCM/MeOH (4:1) mixture at -78 °C.
The UV-visible spectrum of [FeIV=O(TMP+•)]⁺ exhibited characteristic features of the Compound I intermediate, including a broad Soret band at 406 nm and a Q-band at 645 nm. Mössbauer spectroscopy provided evidence of ferromagnetic coupling between the FeIV (S=1) center and the porphyrin π-cation radical spin (1/2), which was consistent with EPR signals observed at g values of 4.3, 3.9, and 2.0, indicating strong ferromagnetic coupling with J > +40 cm⁻¹. An EXAFS study of [FeIV=O(TMP+•)]⁺ revealed a Fe=O distance of 1.6 Å, indicative of a double bond similar to that of the Compound I intermediate.
The Fe=O double bond character was further confirmed by a vibrational mode at 828 cm⁻¹ observed in Raman studies. Since then, various iron (IV)-oxo porphyrin cation radical species have been synthesized with different substituents on the porphyrin core to modify their electronic properties. These species have been fully characterized, and rationalizations have been proposed to correlate their structures with their catalytic activities, some of which will be discussed in the following section.
Notably, a non-heme iron (III) complex featuring a tridentate carbazole ligand can also produce an iron (IV) cation radical species capable of epoxidizing alkenes upon oxidation with the two-electron oxidant PhIO. As with the porphyrin complexes, the π-conjugated system of the carbazole-based ligand is essential for the generation of iron (IV) cation radical species and their catalytic reactivity.
This iron (IV) cation radical species was proposed to be the active intermediate in substrate oxygenation, supported by the detection of an absorption band at 660 nm (ε = 1.4 × 10⁴ M⁻¹·cm⁻¹) typically associated with iron (IV)-oxo porphyrin cation radical species, and a sharp symmetrical EPR signal at g = 2.0 attributed to a π-cation radical in the ligand. However, the electronic structure of this iron (IV) cation radical species warrants further investigation. The observed EPR signal at g = 2 is attributed to the lack of coupling between the FeIV center and the π-cation, contrasting with observations made for porphyrin iron-oxo species.
Over the past two decades, a series of non-heme iron (IV)-oxo species have been synthesized and characterized. The first FeIV=O species, reported in 2000, was generated by ozonolysis of [FeIII(- cyclam-acetato)(CF₃SO₃)]+ at -80 °C in an acetone/water (95:5) mixture. This intermediate is stable for approximately 30 minutes at -80 °C but decomposes rapidly above -40 °C. Mössbauer studies indicated an iron (IV)-oxo species with S = 1, while the EPR spectrum became silent upon oxidation, limiting characterization by other spectroscopic techniques.
Subsequently, using a methyl-substituted cyclam ligand (TMC), which provides an enhanced ligand field compared to cyclam, Que successfully generated an iron (IV)-oxo species stable for at least one month at -40 °C from the reaction of [FeII(TMC)(CH₃CN)]²⁺ with PhIO in acetonitrile. The relative stability of this intermediate enabled its full characterization, particularly through X-ray diffraction spectroscopy. The X-ray structure of [FeIV=O(TMC)(CH₃CN)]²⁺ revealed a terminal Fe-O distance of 1.64 Å, comparable to the average terminal Fe-O double bond distance in K₂FeVIO₄. Since then, several non-heme iron (IV)-oxo species have been synthesized and characterized.
Due to the inherent instability of high-valent iron-oxo species, relatively few iron(V)-oxo species have been prepared and characterized. As previously noted, the formation of a FeIV=O species has been suggested to occur via the degradation of a short-lived FeV=O species. The high oxidative power of FeV=O species is supported by the high potential value of the FeV=O/FeIV-OH redox couple. Consequently, most FeV=O species have been generated and characterized at very low temperatures (-60 to -80 °C), with the exception of [FeV=O(b-TAML)]⁻.
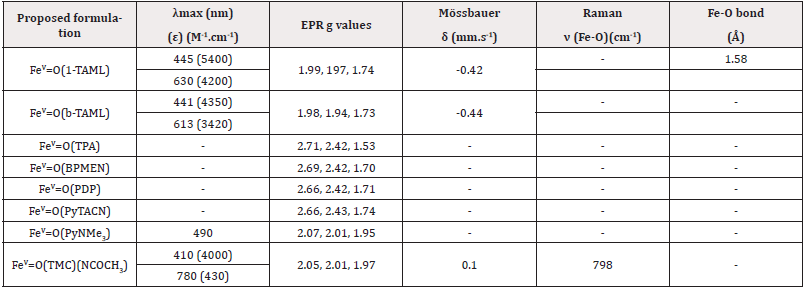
The first iron(V)-oxo species utilizing a strong electron donor ligand, 1-TAML, was reported by Collins in 2007. The high oxidation state of this species was confirmed by a negative isomer shift (δ = -0.46 mm/s) measured by Mössbauer spectroscopy, which is more negative than those of iron (IV)-oxo species previously reported. The EPR signal observed at g values of 1.99, 1.97, and 1.74 was assigned to a low-spin (S = ½) species. Furthermore, EXAFS studies revealed a Fe-O bond distance of 1.58 Å, which is 0.04 to 0.10 Å shorter than those documented for iron (IV)-oxo species. All these data are consistent with the characterization of a low-spin iron (V)-oxo species. To date, no high-spin (S = 3/2) iron(V)-oxo species have been synthesized and characterized, likely due to the strong field produced by the electron donor ligands used in the preparation of iron (V)-oxo species. Unfortunately, no X-ray crystal structures of iron (V)-oxo species have been reported, likely due to their high reactivity in oxidation reactions Table 3.
Interestingly, in the pursuit of Fe=O species, researchers have identified short-lived iron intermediates, denoted as FeO-X (where X represents Cl, OH, O, or OAc), which are recognized as precursors to Fe=O species. Some of these intermediates have been characterized as active species capable of oxygenating substrates.
Conclusion
The exploration of bioinspired iron complexes for Oxygen Atom Transfer (OAT) reactions represents a significant advancement in the quest for sustainable catalytic systems. This research has highlighted the potential of iron-based catalysts to replicate the efficiency and selectivity of natural metalloenzymes, particularly under mild conditions. By leveraging the structural and electronic features inherent in natural systems, we have developed synthetic models that not only facilitate OAT but also demonstrate enhanced stability and reactivity.
Our investigations into the synthesis and characterization of new dipyrrin-based iron complexes have yielded promising results. These complexes have been shown to stabilize high-valent iron-oxo species, crucial intermediates in OAT reactions. Through a combination of spectroscopic techniques, including Mössbauer and EPR spectroscopy, we have elucidated the electronic structures of these species, contributing to a deeper understanding of their reactivity. The ability to generate and characterize these high-valent species is vital for advancing our knowledge of iron-catalysed oxidation processes.
Moreover, the integration of environmentally friendly oxidants, such as dioxygen and water, aligns with the principles of green chemistry, promoting more sustainable methodologies in chemical synthesis. Our studies have demonstrated that these iron complexes can activate dioxygen effectively, producing reactive iron-oxo intermediates that facilitate substrate oxidation. This approach not only reduces reliance on toxic oxidants but also enhances the overall sustainability of the catalytic processes.
The bioinspired strategy adopted in this research has also revealed valuable insights into the mechanisms of OAT reactions. By understanding the fundamental interactions between the iron center and substrates, we can better design ligands that optimize catalytic performance. The incorporation of electron-rich and sterically favourable ligands has been shown to enhance the electrophilicity of iron-oxo species, further improving their reactivity towards various substrates.
In addressing the challenges associated with the instability of high-valent iron-oxo species, we have explored innovative activation strategies that facilitate their generation and utilization. The use of mild reducing agents and controlled reaction conditions has proven effective in stabilizing these reactive intermediates, allowing for their application in catalytic cycles.
As we move forward, the findings from this research will serve as a foundation for the continued development of iron-based catalysts. Future work will focus on expanding the range of substrates that can be efficiently oxidized using these complexes, as well as exploring their potential applications in industrial processes. Additionally, further mechanistic studies will be essential to uncover the intricacies of iron-mediated oxidation, paving the way for the design of next-generation catalysts that can operate with high efficiency and selectivity.
In conclusion, the development of bioinspired iron complexes signifies a promising direction in the field of catalysis, merging the insights gained from nature with synthetic innovation. By continuing to refine our understanding of these systems, we aim to contribute to the broader goal of creating sustainable and efficient catalytic processes that can meet the demands of modern chemical synthesis while minimizing environmental impact. This research not only enhances our comprehension of iron-mediated reactions but also provides a template for future advancements in catalytic science.
Acknowledgement
None.
Conflict of Interest
None.
References
- Thallaj N (2021) Synthesis of a New Ligand Tris (2-pyridylmethyl) amine functionalized by a methoxy group and study of Dichloroferrous complexes, its reactivity to dioxygen both in the presence and absence of substrate. International journal of applied chemistry and biological sciences 2(4): 65-77.
- L Labban, N Thallaj, Z Malek (2020) International Journal of Medical Studies 5(12): 23-36.
- L Labban, M Kudsi, Z Malek, N Thallaj (2020) Pain Relieving Properties of Ginger (Z. officinale) and Echinacea (E.angustifolia) Extracts Supplementation among Female Patients with Osteoarthritis. A Randomized Study. Advances in Medical, Dental and Health Sciences 3(3): 45-48.
- L Labban, N Thallaj, M Al Masri (2020) Journal of Advanced Research in Food Science and Nutrition 3(1): 34-41.
- Thallaj N, agha M I H, nattouf AH, katib CH, karaali A et al. (2020) Evaluation of Antimicrobial Activities and Bioactive Compounds of Different Extracts Related to Syrian Traditional Products of Damask Rose (Rosa damascena). open access library journal 7(5): 1-21.
- L labban, N Thallaj, A labban (2020) Archives of Medicine 12(2): 1-5.
- L labban, N Thallaj (2020) International Journal of Herbal Medicine 8(2): 33-37.
- L Labban, N Thallaj, Z Malek (2019) The implications of E-cigarettes or "vaping"on the nutritional status. Journal of Medical Research and Health Sciences 2(11): 784-787.
- L labban, N Thallaj (2019) The Effect of Magnesium Supplementation on Hba1c Level and Lipid Profile Among Type 2 Diabetics. Acta Scientific Nutritional Health 3(10): 7-12.
- Malek ZS, Labban L (2020) The International Journal of Neuroscience 1-7.
- Malek ZS, Labban L (2019) A Comparative Study of Tryptophan Hydroxylase's Circadian Rhythm in the Functional Parts of Dorsal Raphe Nuclei in the Mesencephalon European Journal of Pharmaceutical and Medical Research 6(11): 527-532.
- Malek ZS (2018) Journal of AlBaath University 40(4): 39-62.
- Malek ZS (2018) Analytical Study for the Determination of Norfloxacin in Pure and Pharmaceutical Formulations using Alizarin by Visible Spectrophotometric Method. Tishreen University Journal for Research and Scientific Studies 40(2).
- ZS Malek, LM Labban (2021) Photoperiod regulates the daily profiles of tryptophan hydroxylase-2 gene expression the raphe nuclei of rats. International Journal of Neuroscience 131(12): 1155-1161.
- ZS Malek, LM Labban (2020) Photoperiod regulates the daily profiles of Tryptophan Hydroxylase-2 gene expression the raphe nuclei of rats. Journal of current research in physiology and pharmacology 4(1): 1-5.
- LM Labban, MM Alshishkli, A Alkhalaf, Z Malek (2017) J Adv Res Dent Oral Health 2(3&4): 1-4.
- L Labban, ZS Malek (2018) Open Access Library Journal 5(07): 1-11.
- L Labban, ZS Malek (2019) Ann Food Nutr Res J 1: 1
- Labban L, N Thallaj (2019) Acta Scient Nutr Health 3: 7-12.
- N Thallaj (2022) Tishreen university journal 44(1): 59-77.
- N Thallaj (2022) Tishreen university journal 44(2): 87-105.
- A Abbood, N Thallaj (2023) Arab Journal of Pharmaceutical Sciences 7(1).
- N Thallaj (2023) Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- Machkour A, Thallaj NK, Benhamou L, Lachkar M, Mandon D (2006) The coordination chemistry of FeCl3 and FeCl2 to bis[2-(2,3-dihydroxyphenyl)-6-pyridylmethyl] (2-pyridylmethyl) amine: access to a diiron (III) compound with an unusual pentagonal-bipyramidal/square-pyramidal environment. Chemistry 12(25): 6660-
- Thallaj N, Machkour A, Mandon D, Welter R (2005) Square pyramidal geometry around the metal and tridentate coordination mode of the tripod in the [6-(3′-cyanophenyl)-2-pyridylmethyl] bis(2-pyridylmethyl) amine FeCl2 complex: a solid-state effect New J Chem 29: 1555 - 1558.
- Thallaj NK, Rotthaus O, Benhamou L, Humbert N, Elhabiri M (2008) Reactivity of molecular dioxygen towards a series of isostructural dichloroiron(III) complexes with tripodal tetraamine ligands: general access to mu-oxodiiron(III) complexes and effect of alpha-fluorination on the reaction kinetics. Chemistry-A European Journal 14(22): 6742-6753.
- Wane A, Thallaj NK, Mandon D (2009) Biomimetic Interaction between FeII and O2: Effect of the Second Coordination Sphere on O2 Binding to FeII Complexes: Evidence of Coordination at the Metal Centre by a Dissociative Mechanism in the Formation of μ-Oxo Diferric Complexes. Chemistry 15(40): 10593-10602.
- Thallaj NK, Orain PY, Thibon A, Sandroni M, Welter R (2014) Steric congestion at, and proximity to, a ferrous center leads to hydration of α-nitrile substituents forming coordinated carboxamides. Inorg Chem53(15): 7824-7836.
- NK Thallaj, J Przybilla, R Welter, D Mandon (2008) A Ferrous Center as Reaction Site for Hydration of a Nitrile Group into a Carboxamide in Mild Conditions. J Am Chem Soc 130: 2414-2415.
- NK Thallaj, D Mandon, KA White (2007) The Design of Metal Chelates with a Biologically Related Redox-Active Part: Conjugation of Riboflavin to Bis(2-pyridylmethyl) amine Ligand and Preparation of a Ferric Complex. Eur J of Inorg Chem 2007: 44-47.
- Thallaj N (2021) Synthesis of a New Ligand Tris (2-pyridylmethyl) amine functionalized by a methoxy group and study of Dichloroferrous complexes, its reactivity to dioxygen both in the presence and absence of substrate. International journal of applied chemistry and biological sciences 2(4): 65-77.
- Thallaj N (2023) Review of a Few Selected Examples of Intermolecular Dioxygenases Involving Molecular Oxygen and Non-Heme Iron Proteins. Int J Adv Parmacutical Sci Res (IJAPSR) 3: 1-18.
- L Labban, M Kudsi, Z Malek, N Thallaj (2020) Pain Relieving Properties of Ginger (Z. officinale) and Echinacea (E. angustifolia) Extracts Supplementation among Female Patients with Osteoarthritis. A Randomized Study. Advances in Medical, Dental and Health Sciences 3(3): 45-48.
- L Labban, N Thallaj, M Al Masri (2020) The Nutritional Value of Traditional Syrian Sweets and Their Calorie Density. Journal of Advanced Research in Food Science and Nutrition 3(1): 34-41.
- L labban, N Thallaj, A labban (2020) Assessing the Level of Awareness and Knowledge of COVID 19 Pandemic among Syrians. archives of medicine 12(2): 1-5.
- L Labban, N Thallaj, Z Malek (2019) The implications of E-cigarettes or "vaping “on the nutritional status. Journal of Medical Research and Health Sciences 2(11): 784-787.
- Malek ZS, Sage D, Pevet P, Raison S (2007) Daily Rhythm of Tryptophan Hydroxylase-2 Messenger Ribonucleic Acid within Raphe Neurons Is Induced by Corticoid Daily Surge and Modulated by Enhanced Locomotor Activity. Endocrinology 148 (11): 5165-5173.
- Malek ZS, Dardente H, Pevet P, Raison S (2005) Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. European Journal of Neuroscience 2005 22(4): 895-901.
- A Abbood, SA Malik, D aldiab, HH Ali, N Thallaj (2025) Investigation of the charge variant profile of non-cleavable conjugated antibodies. Research J Pharm and Tech 18(1): 185-190.
- Malek S, Pevet P, Raison S (2004) Neuroscience 125(3): 749-758.
- Malek ZS, Labban L (2020) The International Journal of Neuroscience :1-7.
- ZS Malek, LM Labban (2020) Journal of current research in physiology and pharmacology 4(1): 1-5.
- LM Labban, MM Alshishkli, A Alkhalaf, Z Malek (2017) J Adv Res Dent Oral Health 2(3&4): 1-4.
- L Labban, ZS Malek, Open Access Library Journal, 2018, 5 (07), 1-11.
- Y alhomush, Z malek, A Abboud, N Thallaj (2022) Research Journal of Pharmacy and Technology 15: 10.
- A Abbood, Z Malek, N Thallaj (2022) Research Journal of Pharmacy and Technology 15(11): 4935-4939.
- Thallaj N, agha MIH, nattouf AH, katib CH, karaali A, Moustapha A, et al. (2020) open access library journal 7(5): 1-21.
- N Thallaj (2021) Indian journal of advanced chemistry 1(2): 20-26.
- N Thallaj (2022) Indian journal of advanced chemistry 2(2): 1-11.
- N Thallaj (2022) Indian journal of advanced chemistry 2(1): 5-9.
- N Thallaj (2022) Indian journal of advanced chemistry 2(1): 10-14.
- N Thallaj, Xi'an ShiyouDaxueXuebao (ZiranKexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(06): 289-301.
- N Thallaj, Xi'an ShiyouDaxueXuebao (ZiranKexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(06): 313-328.
- Z MALEK, A ABBOOD, N THALLAJ, Xi'an ShiyouDaxueXuebao (ZiranKexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(06): 302-312.
- N Thallaj, Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(7): 169-184.
- Z MALEK, Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(7): 143-152.
- Thallaj, Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(7): 110-142.
- N Thallaj (2023) Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(3): 1-28.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(4): 1-15.
- N Thallaj (2023) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(2): 1-18.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(6): 1-12.
- N Thallaj (2023) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(3): 1-10.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(1): 32-52.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(5): 29-49.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(4): 7-21.
- N Thallaj. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2024.4, 6,7-27.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR)4(6): 33-48.
- Besherb S, Alallan L, Hassan Agha MA, AIshamas I, Thallaj N, et al. (2024) Influence of soil salinity on the chemical composition of essential oil of Rosmarinus Officinalis in Syria, Research J. Pharm. and Tech 17(5).
- Thallaj N (2024) Advancements in Pharmaceutical Science: Synthesis and Application of Molecular Cages Integrating N-Heterocyclic Carbenes for Enhanced Stability and Functionality. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(1): 6-19.
- Ayat Abbood, Hassan Hadi Ali, Samir Azzat Malik, Dima AlDiab, Nasser Thallaj, et al. (2025) Investigation of the Charge Variant Profile of Non-cleavable Conjugated Antibodies. Research Journal of Pharmacy and Technology 18(1): 185-190.
- Thallaj N (2025) Analyzing Charge Variant Profiles of Monoclonal Antibodies Conjugated to Cytotoxic Agents. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(3): 20-26.
- Thallaj N (2025) Biomimetic Synthesis and Phytochemical Analysis of Lodopyridone: Insights into 4-Pyridone Derivatives and Thiopeptide Antibiotic. Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(3): 9-19.
- Mousa Al Khleif, Nour Alhuda Alzoubi, Ghassan Shannan, Zeina S Malek, Nasser Thallaj (2025) Exploring Circadian Rhythms:A Comprehensive Study on Sleep Patterns and Disorders in Syrian Society. Am J Biomed Sci & Res 26(2).
- Ranim Abdul Rahman, Louai Alallan, Ahmed Khalid Aldhalmi, Nasser Thallaj (2025) Separation, Determination, and Potential Application of Active Compounds in Porosopis cineraria: A Comprehensive Analysis. Research Journal of Pharmacy and Technology 18(4):1604-1610.
- Dalia Aboufakher, Rita Zeinaldin, Racha Khatib, Rawa Khreit, Mohamed Sami Joha, et al. (2025) Prevalence and AntibioticResistance Patterns of Multidrug-Resistant Gram-Negative Bacilli in Hospitalized Patients in Sweida, Syria. Am J Biomed Sci & Res. 2025 26(3): 309-315
- Besher S, Alallan L, Hasan Agha MI, Alshamaa I, Thallaj N (2024) Influence of Soil Salinity on the Chemical Composition of Essential Oil of Rosmarinus officinalis in Syria. Research Journal of Pharmacy and Technology 17(5): 2282- 2288.
- Khatib O, Alshimale T, Alsaadi A, Thallaj N (2024) The Global Impact of HIV: A Comprehensive Review. IJAPSR 4(3): 6-19.
- Samer alkhoury, Rasha Kateeb, Rawa Akasha and Nasser Thallaj (2025) Analysis of Crocin Content in Saffron (Crocuss ativus L) Cultivated in Syria Using Liquid Chromatography-Mass Spectrometry. Am J Biomed Sci & Res. 2025 26(3).
- Zanboua R, Abbood A (2024) Survey of Knowledge About the Interaction Between Food and Drugs Among the Syrian Population. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(4): 22-28.
- Qattan M, Dashash M, S Malek Z (2024) Enhancing Academic Success: A mixed Study on the Influencing Factors among Pharmacy Students in Syrian Universities. F1000Res 13: 868.
- Thallaj N (2022) Design and Synthesis Ligands Tetradents Substituted with Halogenes in α- Position and Conjugation with Riboflavin (Bioconjugates): Conjugate ligands Type TPA’s with Flavonoids as un–Electron Mediator. Biomedicine and Chemical Sciences, 1(2): 47-56.
- Thallaj N, Alrasho JF, Sofi FK (2024) Advancements in Antiviral Therapeutics: A Comprehensive Review of Hepatitis C Virus and Novel Flavone Leads. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(1): 28-40.
- Thallaj N (2025) Analyzing Charge Variant Profiles of Monoclonal Antibodies Conjugated to Cytotoxic Agents. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(3): 20-26.
- Thallaj N (2025) Biomimetic Synthesis and Phytochemical Analysis of Lodopyridone: Insights into 4-Pyridone Derivatives and Thiopeptide Antibiotics. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(3): 9-19.
- Ghassan Shannan, Zeina S (2025) Malek and Nasser Thallaj. Pactamycin: A Comprehensive Review of Its Biological Activity, Biosynthesis, And Synthetic Strategies in the Fight Against Antibiotic Resistance. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12(4): 334-353.
- Ghassan Shannan, Zeina S (2025) Malek and Nasser Thallaj. A Review of Antibiotic-Induced Drug Allergies: Mechanisms, Prevalence, And Future Perspectives. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12(4): 387-399.
- Alma Soudan, Joudy Abokassem, Shaam Ammar and Nasser Thallaj (2025) Advancements in the Total Synthesis of Cyclotripeptidic Natural Products: Exploring the Therapeutic Potential Of Marine-Derived Compounds. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12(4): 354-377.
- Labban L, Thallaj N, Labban A (2020) Assessing the level of awareness and knowledge of COVID 19 pandemic among syrians. Arch Med 12(2): 8.
- Labban L, Kudsi M, Malek Z, Thallaj N (2020) Advances in Medical. Dental and Health Sciences 3(3): 45-48.
- Thallaj N (2021) Efficiency in transporting molecular oxygen to iron (II) complexes with ligands type tri (2-pyridylmethyl) amine substitution aromatic in (α) position by a mechanism that mimics biological oxidation.
- Labban L (2020) International Journ. IJMS 5(12): 23-36.
- Thallaj N (2022) Tishreen University Journal-Medical Sciences Series 44(1): 59-77.
- Thallaj N (2020) Evaluation of antimicrobial activities and bioactive compounds of different extracts related to syrian traditional products of damask rose (Rosa damascena). Open Access Library Journal 7(05): 1.
- Thallaj N (2023) Characterization of charge heterogeneity of antibody-drug conjugate by anion-exchange chromatofocusing. Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- Labban L, M Kudsi, Z Malek, N Thallaj (2020) Advances in Medical, Dental and Health Sciences 3(3): 45-48.
- Thallaj N (2023) A Brief Overview of the General Characteristics and Reactivity Towards Dioxygen of the Ferrous Tris (2-Pyridylmethyl Amine) Series Complexes is Presented. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(3): 1-18.
- Abbood A, Thallaj N (2023) Comparison between chromatofocusing and icIEF charge variant profiles of unconjugated monoclonal antibodies and their drug conjugates. Arab Journal of Pharmaceutical Sciences 7(1).
- Thallaj N (2024) Detecting Antioxidant Behavior for Phenolic Content of Some Beauty Care Creams in Syrian Market. Indian Journal of Advanced Chemistry 2(1): 10-14.
- Thallaj N (2024) Advancements in Pharmaceutical Science: Synthesis and Application of Molecular Cages Integrating N-Heterocyclic Carbenes for Enhanced Stability and Functionality. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(1): 6-19
- Thallaj N (2023) Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- Thallaj N (2024) Conductive Nanocomposites Based on Graphene and Natural Polymers. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(6): 7-27.
- Thallaj N (2022) Design and Synthesis Ligands Tetradents Substituted with Halogenes in α-Position and Conjugation with Riboflavin (Bioconjugates): Conjugate ligands Type TPA’s with Flavonoids as un Electron Mediator. Biomedicine and Chemical Sciences 1(2): 47-56.
- Dayoub L, Alkuddsi Y, Thallaj N (2023) Investigating the interaction between some of Bipolaris sorokiniana’s toxins and the Gα Subunit of the Wheat G-Protein using bioinformatics tools. University of Thi-Qar Journal of agricultural research 12(1): 181-200.
- Thallaj N, Abbood A (2023) Tishreen University Journal-Medical Sciences Series 45: 47-57.
- Thallaj N (2022) HPLC method validation for determination of pentoxifylline in pharmaceutical dosage forms. Indian J Adv Chem 2(1): 5-9.
- Thallaj N (2022) Ligands tris (2-pyridylmethyl) amine with nitrile group in α-substituted and the corresponding FeCl2 complexes.
- Thallaj N (2022) Review of Calixarene-Derivatives in Transition Metal Chemistry. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(3): 1-30.
- Thallaj N (2022) Ligands tris (2-pyridylmethyl) amine with nitrile group in α-complexes 2 substituted and the corresponding FeCl. Journal of Biological Pharmaceutical And Chemical Research 9(1): 25-47
- Thallaj N (2022) Journal of Biological Pharmaceutical And Chemical Research 9 (3): 40-47.
- NK Thallaj (2018) Damascus University Journal for Basic Sciences. Conjunction of riboflavin to TPAs ligands with two halogens atoms in α-position substitution 34: 1.
- NK Thallaj (2017) Designing and synthesis of DPAs ligands to induce structural modification of Riboflavin. Journal of AlBaath University 39(8): 33-72.
- THALLAJ, Nasser Ferrous complexes with aromatic α-substituted tris (2-pyridylmethyl) amine ligands: effect of the substituents and biomimetic reactivity.
- Thallaj N (2024) Advancements in Peptide Vectors for Cancer Therapy and Tumor Imaging: A Comprehensive Review. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(5): 29-49.
- Thallaj N, ali Hamad DH, Batieh NA, Saker GA (2023) A Review of Colon Cancer Treatment using Photoactive Nanoparticles. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(4): 1-32.
- Labban, Louay (2020) International Journ. IJMS 5(12): 23-36.
- Labban L, Malek Z (2020) Photoperiod regulates the daily profiles of tryptophan hydroxylase-2 gene expression the raphe nuclei of rats. International Journal of Current Research in Physiology and Pharmacology 1-5.
- Labban, Louay, Nasser Thallaj, and Zeina Malek (2019) The implications of E-cigarettes or" vaping" on the nutritional status. Jour Med Resh and Health Sci 2(11): 784-787.
- MALEK Z (2018) The effect of regular exercise on the expression of tryptophan hydroxylase-2 gene within Raphe complex: functional relationship with adrenal hormones and glucose blood levels. in Journal of AlBaath University.
- MALEK Z (2018) The effect of Glucocorticoids rhythm on serotonin release within the hypothalamic suprachiasmatic nuclei; the locus of the Biological Clock: functional relationship with blood glucose. Tishreen University Journal for Research and Scientific Studies 40: 1-17.
- Labban L (2018) The Association between Visceral Fat, Dietary Patterns, and Comorbidities. Open Access Library Journal 5(07): 1.
- Labban LM, Alshishkli MM, Alkhalaf A, Malek Z (2017) The effects of dental amalgam toxicity on health and nutritional status. J. Adv. Res. Dent. Oral Health 2: 1-4.
- Shannan G, Malek ZS, Thallaj N (2025) Advancements in Protein Synthesis: Triazole Multi-Ligation and Cycloadditions for Pharmaceutical Applications. Am J Biomed Sci & Res 26(5): 685-700.
- Channan G (2018) Communicable diseases in the Mediterranean region. EJIFCC 29(3): 164-170.
- Shannan G (2011) TUBERCULOSIS IN THE AFCB COUNTRIES; RE-EMERGING DISEASE. In CLINICAL CHEMISTRY AND LABORATORY MEDICINE. GENTHINER STRASSE 13, D-10785 BERLIN, GERMANY: WALTER DE GRUYTER & CO 49: S41-S41.
- Channan GM (1977) Studies of vitamin D hydroxylation in chronic renal failure.
- Aldakhoul M, Sleman S, Alragheb N, Alfarwan M, Alallan L, et al. (2024) Phytochemical Composition and Health Benefits of Pumpkin. Research Journal of Pharmacy and Technology 17(10): 4915-4921.
- Khabbazeh Sally, Saly Alhaddad, Yazan Kiwan, Louai Alallan and Nasser Thallaj, et al. (2024) The Benefits of Jujube: Extracting Phenols and Saponins for Medicinal and Nutritional Uses." Azerbaijan Pharmaceutical and Pharmacotherapy Journal 23(4): 1-7.
- Nasser Thallaj, Dalia Aboufakher, Rita Zeinaldin, Rawa Khreit, Racha Khatib (2025) Prevalence and Antibiotic Resistance Patterns of Multidrug-Resistant Gram-Negative Bacilli in Hospitalized Patients in Sweida, Syria. FJHMS 1(1): 70-78.
- Thallaj Dr N (2022) Review of Calixarene-Derivatives in Transition Metal Chemistry. International Journal of Advanced Pharmaceutical Sciences and Research 2(3): 1-28.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.